Environment-friendly clean method for preparing dimethyl malonate
A dimethyl malonate, clean technology, applied in the preparation of carboxylate, the preparation of organic compounds, chemical instruments and methods, etc., can solve the problems of easy decomposition of cyanoacetic acid, consumption of sulfuric acid acid waste water, etc., to avoid cyanoacetic acid The effect of decomposition and reduction of waste water generation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
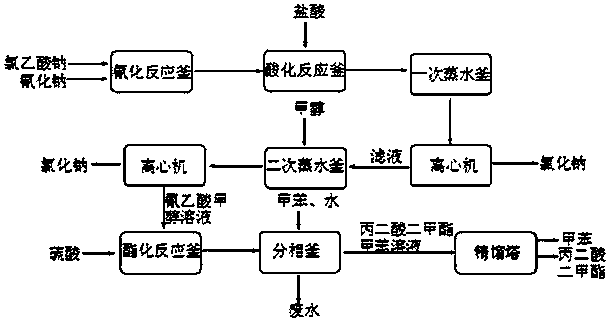
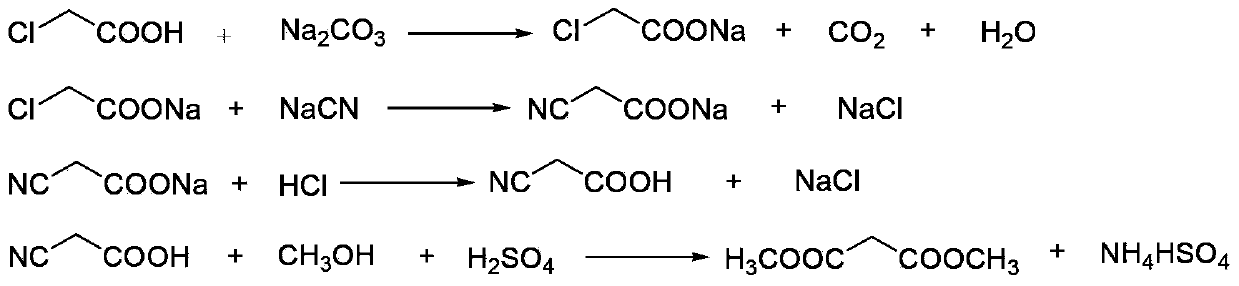
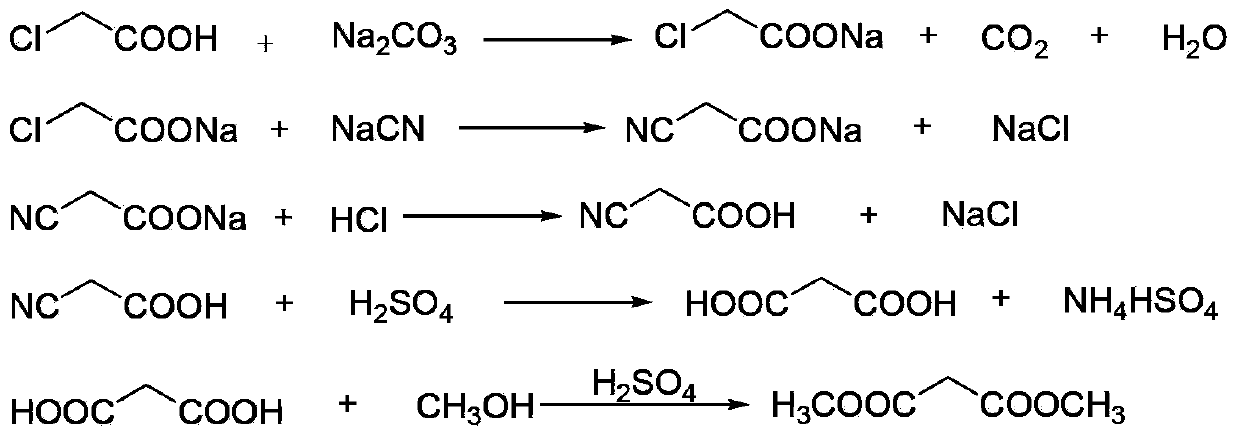
[0026] The following will refer to figure 1 , further illustrate the present invention by embodiment:
[0027] (1) Neutralization reaction: add 900kg of chloroacetic acid (content 98%, 9.30kmol) to 620kg of water, stir and dissolve, neutralize with aqueous sodium carbonate to pH=6.5-7.0, and generate sodium chloroacetate;
[0028] (2) Cyanide reaction: transfer the sodium chloroacetate obtained in step (1) into the cyanide reaction kettle, raise the temperature to 50°C, then add 1530kg (4.69kmol) of 30% sodium cyanide aqueous solution dropwise, when the reaction temperature reaches 85 After -90°C, stop heating and start to cool. When the reaction temperature reaches 105°C, the reaction ends and is cooled to room temperature to obtain an aqueous solution of sodium cyanoacetate;
[0029] (3) Acidification reaction: transfer the sodium cyanoacetate aqueous solution obtained in step (2) into the acidification reaction kettle, stir, and slowly add 1000L (content, 31%, 10.14kmol) h...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com