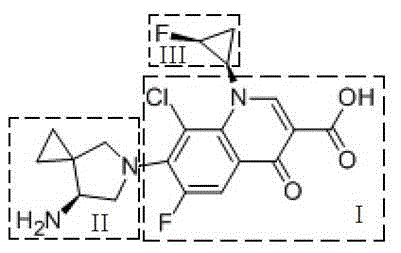
Preparation method for three-membered ring intermediate of sitafloxacin hydrate
A sitafloxacin and three-membered ring technology, which is applied in the field of preparation of sitafloxacin three-membered ring intermediates, can solve the problems of cis-trans isomerism and low overall yield level, and achieve high product yield and purity, It is easy to scale up to the effect of large-scale production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0027] A preparation method of sitafloxacin three-membered ring intermediate, comprising the steps of:
[0028] (1) Construction of a three-membered ring: Dissolve dimethyl malonate and 1,1,2-tribromo-2-fluoroethane in anhydrous dimethylformamide, add anhydrous potassium carbonate in batches, and Heating in a water bath at 25-35°C for 55-60 hours, the obtained solution was evaporated to dryness to obtain solid A;
[0029] (2) Debromination: Dissolve solid A in methanol, use palladium carbon as a catalyst to react with hydrogen gas for 20-24 hours, filter, and evaporate the solvent to obtain oily liquid B;
[0030] (3) Debranching: Dissolve the oily liquid B in a mixture of dimethylformamide and saline, reflux for 30-35 hours, evaporate the solvent to dryness, and obtain solid C;
[0031] (4) Hydrolysis: Dissolve solid C in a mixture of tetrahydrofuran and water and place it in an ice-water bath, stir and add lithium hydroxide monohydrate, then add hydrochloric acid dropwise t...
Embodiment 1
[0038] (1) Take 1.32kg dimethyl malonate and 3.7kg 1,1,2-tribromo-2-fluoroethane into a 50L three-necked flask, dissolve with 15L anhydrous dimethylformamide, and place the three-necked flask In a water bath at 25°C, add 4.4kg of anhydrous potassium carbonate in batches under uniform stirring and react for 60 hours. After the reaction, add 30L of ice water first, then extract with ethyl acetate (8L×5), combine the organic phases, and separate the organic The phase was washed with saturated aqueous sodium chloride until neutral, then dried over anhydrous sodium sulfate, and the solvent was evaporated to dryness. Then, the residue was added to 3 L of diethyl ether, stirred for 4 hours under ice bath, and finally 1.7 kg of solid A was obtained by filtration.
[0039] (2) Take the obtained solid A and put it into a 10L high-pressure hydrogenation kettle, add 6L of methanol to dissolve, then add 72g of palladium carbon as a catalyst, and replace the air in the kettle with hydrogen f...
Embodiment 2
[0047] (1) Take 1.32kg dimethyl malonate and 3.7kg 1,1,2-tribromo-2-fluoroethane into a 50L three-necked flask, dissolve with 15L anhydrous dimethylformamide, and place the three-necked flask In a water bath at 35°C, add 4.4kg of anhydrous potassium carbonate in batches under uniform stirring and react for 55 hours. After the reaction, add 30L of ice water, then extract with ethyl acetate (8L×5), combine the organic phases, and separate The phase was washed with saturated aqueous sodium chloride until neutral, then dried over anhydrous sodium sulfate, and the solvent was evaporated to dryness. Then, the residue was added to 3 L of ether, stirred for 4 hours under ice bath, and finally 1.81 kg of solid A was obtained by filtration.
[0048] (2) Take the obtained solid A and put it into a 10L high-pressure hydrogenation kettle, add 6L of methanol to dissolve, then add 72g of palladium carbon as a catalyst, and replace the air in the kettle with hydrogen for 4 times, then continue...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com