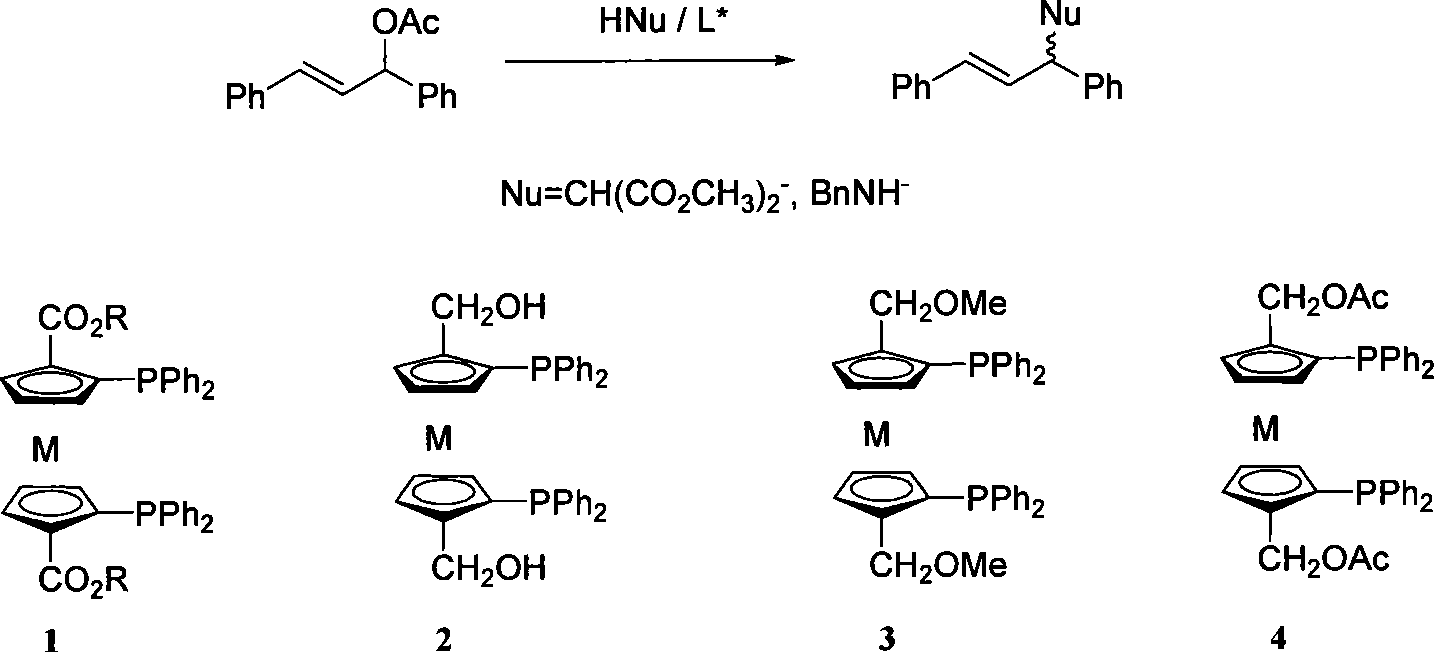
Enantioselectivity reversion method for allyl substitution reaction catalyzed by Pd
An enantioselective, allyl substitution technology, applied in the field of enantioselective inversion, can solve the problems of poor configuration prediction and low ee value, and achieve the effect of simple operation and wide range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Alkylation reaction
[0020] Under nitrogen atmosphere, chiral ligand (0.03mmol) and [Pd(η 3 -C 3 h 5 )Cl] 2 (4.60 mg, 12.5 μmol) was dissolved in dry acetonitrile (1 mL) and stirred at room temperature for 1 h. The obtained in-situ catalyst was added into another solution by syringe, which was dissolved in 1,3-diphenylallyl acetate (0.252 g, 1.00 mmol) and anhydrous potassium acetate (0.0020 g, 20 μmol) Dry acetonitrile (2 mL) was formed. Then, dimethyl malonate (0.396 g, 3.00 mmol) and BSA (0.613 g, 3.00 mmol) were added in sequence, and the reaction was monitored by TLC, and the reaction was completed after 0.5 h. Dilute the system (20mL) with diethyl ether, wash with cold saturated NH 4 Cl aqueous solution (25mL), water and saturated brine washing, anhydrous Na 2 SO 4 After drying, the solvent was evaporated under reduced pressure. The residue was obtained by column chromatography (ethyl acetate / petroleum ether=1 / 8). ee value measured on chiral OD-H column...
Embodiment 2
[0023] Alkylation reaction
[0024] Under nitrogen atmosphere, chiral ligand (0.03mmol) and [Pd(η 3 -C 3 h 5 )Cl] 2 (4.60 mg, 12.5 μmol) was dissolved in dry dichloromethane (1 mL) and stirred at room temperature for 1 h. The obtained in-situ catalyst was added into another solution by syringe, which was dissolved in 1,3-diphenylallyl acetate (0.252 g, 1.00 mmol) and anhydrous potassium acetate (0.0020 g, 20 μmol) Dry dichloromethane (2 mL) formed. Cool down to -78°C, then add dimethyl malonate (0.396g, 3.00mmol) and BSA (0.613g, 3.00mmol) in sequence, and monitor the completion of the reaction by TLC. Dilute the system (20mL) with diethyl ether, wash with cold saturated NH 4 Cl aqueous solution (25mL), water and saturated brine washing, anhydrous Na 2 SO 4 After drying, the solvent was evaporated under reduced pressure. The residue was obtained by column chromatography (ethyl acetate / petroleum ether=1 / 8). ee value measured on chiral OD-H column: 72.9%
[0025] 1 H...
Embodiment 3
[0027] Amination reaction
[0028] Under nitrogen atmosphere, chiral ligand (0.015mmol) and [Pd(η 3 -C 3 h 5 )Cl] 2 (2.3 mg, 0.0063 mmol) was dissolved in dry toluene (1 mL) and stirred at room temperature for 1 h to prepare the in situ catalyst. To this was added a solution of 1,3-diphenylallyl acetate (126 mg, 0.5 mmol) and dry toluene (1 mL). After 10 min, benzylamine (131 μL, 1.5 mmol) was added, the reaction was monitored by TLC, and the reaction was completed after 20 min. Dilute the system (20mL) with diethyl ether, wash with cold saturated NH 4 Cl aqueous solution (25mL), water and saturated brine washing, anhydrous Na 2 SO 4 After drying, the solvent was evaporated under reduced pressure. The residue was obtained by column chromatography (ethyl acetate / petroleum ether=1 / 10). Chiral OJ-H column measurement ee value: 63.1 ~ 70.1%.
[0029] 1 H NMR (400MHz, CDCl 3 ): δ3.75-3.81 (m, 2H), 4.41 (d, J = 7.6Hz, 1H), 6.32 (dd, J = 7.6, 15.6Hz, 1H), 6.58 (d, J = 16H...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com