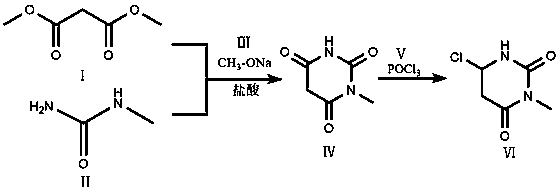
Preparation method of 6-chlorine-3-methyluracil
A technology of methyluracil and methylurea, which is applied in the field of biopharmaceuticals, can solve the problems of less than 60% overall yield, environmental pollution of large methyl iodide, and low yield of synthesis process, and achieve simple reaction process and safety Good, high yield and high purity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
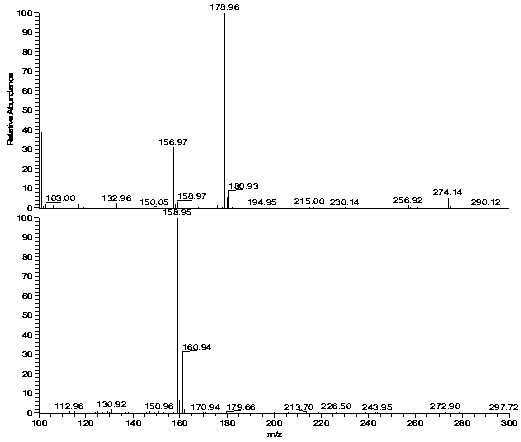
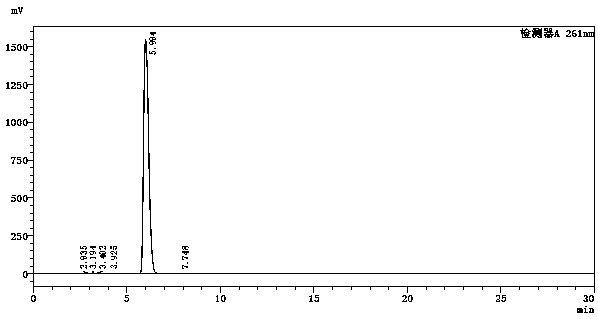
Image
Examples
Embodiment 1
[0041] Preparation of 1-methylbarbituric acid solid: Add 15g methylurea II and 11g sodium methoxide III to 500ml methanol solution, adjust the temperature to 50°C; keep the temperature, slowly add 24ml dimethyl malonate I dropwise for 2 hours; After the dropwise addition, the temperature was raised to 60°C for reflux reaction to generate 1-methylbarbituric acid IV; the reactant was cooled to 30°C, and the methanol was concentrated so that the total amount of methanol was concentrated to 60% of the total amount added initially, and 1-methylbarbituric acid IV was precipitated. Methyl barbituric acid solid IV crystals, filtered and dried;
[0042] Purification of 1-methylbarbituric acid crystals: control the temperature at 20°C, add hydrochloric acid to the dried 1-methylbarbituric acid solid IV crude product, the mass ratio of crystals to hydrochloric acid is 1:2.5; maintain the temperature Stir at 20°C, stir, centrifuge, and dry to obtain 1-methylbarbituric acid IV as a solid; ...
Embodiment 2
[0046] Preparation of 1-methylbarbituric acid solid: add 15g methylurea II and 11g sodium methoxide III to 500ml methanol solution, adjust the temperature to 40°C; keep the temperature, slowly add 24ml dimethyl malonate I dropwise for 1 hour; After the dropwise addition, the temperature was raised to 60°C for reflux reaction to generate 1-methylbarbituric acid IV; the reactant was cooled to 20°C, and the methanol was concentrated so that the total amount of methanol was concentrated to 80% of the total amount added initially, and 1-methylbarbituric acid IV was precipitated. Methyl barbituric acid solid IV crystals, filtered and dried;
[0047] Purification of 1-methylbarbituric acid crystals: control the temperature at 10°C, add hydrochloric acid to the dried 1-methylbarbituric acid solid IV crude product, the mass ratio of crystals to hydrochloric acid is 1:1.5; maintain the temperature Stir at 10°C, stir, centrifuge, and dry to obtain 1-methylbarbituric acid IV as a solid; t...
Embodiment 3
[0051] Preparation of 1-methylbarbituric acid solid: Add 15g methylurea II and 11g sodium methoxide III to 500ml methanol solution, adjust the temperature to 45°C; keep the temperature, slowly add 24ml dimethyl malonate I dropwise for 1.5h After the dropwise addition, the temperature was raised to 60°C for reflux reaction to generate 1-methyl barbituric acid IV; the reactant was cooled to 25°C, concentrated methanol, and the total amount of methanol was concentrated to 70% of the total amount added initially, and 1 -Methyl barbituric acid solid IV crystals, filtered and dried;
[0052] Purification of 1-methylbarbituric acid crystals: control the temperature at 15°C, add hydrochloric acid to the dried 1-methylbarbituric acid solid IV crude product, the mass ratio of crystals to hydrochloric acid is 1:2; maintain the temperature Stir at 15°C, stir, centrifuge, and dry to obtain 1-methylbarbituric acid IV as a solid; the yield is 90%, and the purity is 98%.
[0053] Preparation...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com