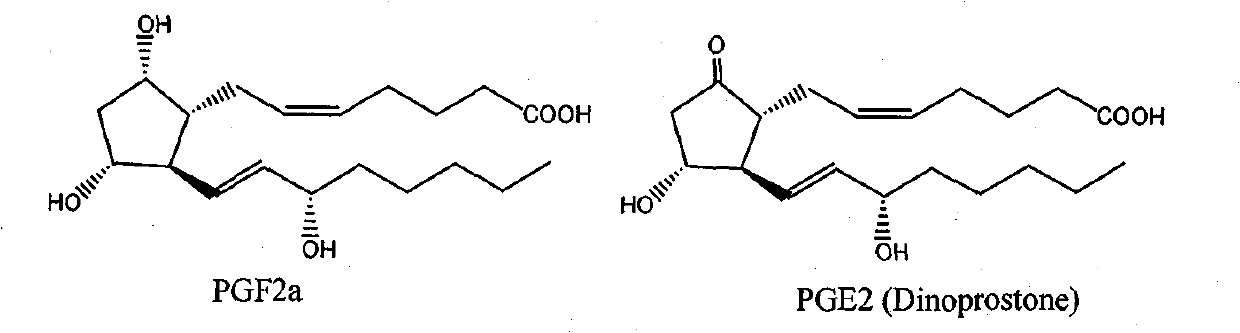
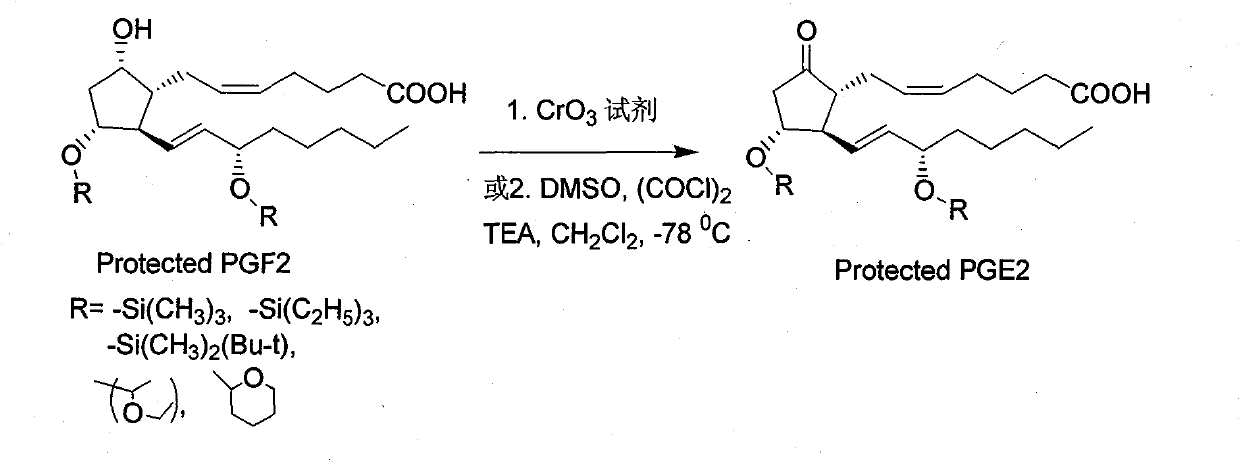
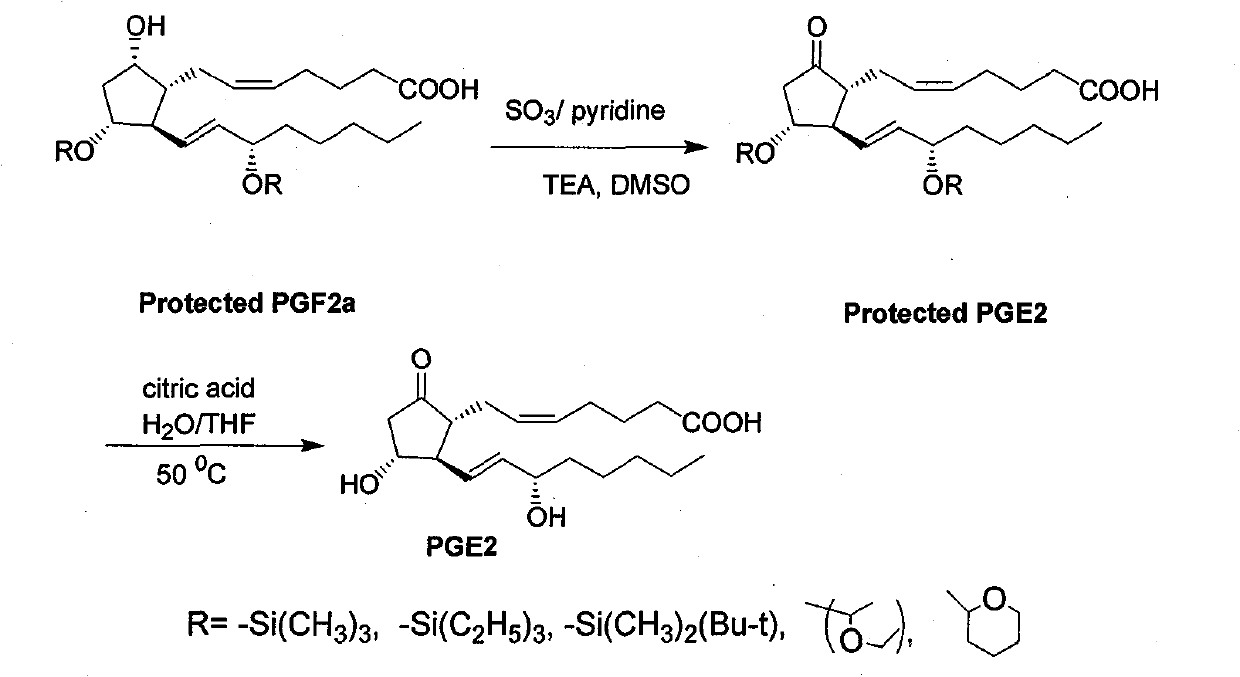
Green synthesis method for dinoprostone (prostaglandin PGE2)
A dinoprostone and prostaglandin technology, applied in the production of bulk chemicals, organic chemistry, etc., can solve the problems of high equipment requirements, harsh conditions, unstable Swern reaction process, etc., and achieve convenient and easy post-processing, mild and fast reaction. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1: Oxidation of 11,15 hydroxyl protected PGF2α
[0032]Add 12 grams (0.075 moles) of pyridine sulfur trioxide and 50 milliliters of DMSO into a flask, and stir to dissolve it with electromagnetic stirring. In another four-neck flask equipped with a mechanical stirrer, a thermometer, and a constant pressure dropping funnel, add respectively 12.5 grams (0.025mol) of PGF2α, 25 milliliters of DMSO, and 23 milliliters of triethylamine, and the whole process system is protected with nitrogen. Keep stirring, and the temperature is controlled between 20-25°C. Then the prepared sulfur trioxide pyridine-DMSO solution is transferred to the dropping funnel and slowly dripped into the reaction flask, controlling the temperature between 20-25°C. After the addition, stir at 20-25°C for 15 minutes, follow the reaction progress by TLC until the starting point disappears, and the reaction usually ends within 30 minutes. In another beaker, add 75 grams of citric acid and 180 mil...
Embodiment 2
[0033] Example 2: Preparation of PGE2 by hydrolysis and deprotection
[0034] Add 12.4 grams (0.025 moles) and 65 milliliters of tetrahydrofuran to a 500-milliliter four-neck flask equipped with a mechanical stirrer, a reflux condenser, and a thermometer vacuum / nitrogen switch to remove oxygen. After stirring and dissolving, add 26 milliliters of 50% citric acid aqueous solution . The reaction solution was stirred and hydrolyzed at 50° C. for 1-2 hours. Samples were taken every 30 minutes and detected by TLC until the raw material point disappeared. After the reaction is completed, cool to 20-25° C., add 90 ml of water and adjust the pH to 3.6-4.0 with 10% aqueous sodium bicarbonate (about 84 g). Next, 95 ml of ethyl acetate was added, and after stirring well, the mixture was allowed to stand and the layers were separated. After the upper organic phase was separated, the aqueous layer was extracted with 50 ml of ethyl acetate, and the organic phases were combined. After th...
Embodiment 3
[0035] Embodiment 3: the crystallization and recrystallization refining of PGE2
[0036] Add 12.5 grams of PGE2 crude product and 50 milliliters of ethyl acetate to a 250 milliliter four-necked flask, and control the temperature at 20-25° C., add 1.25 grams of activated carbon, and stir for 1 hour. After filtering, the filtrate was distilled under reduced pressure, and the oily residue after removing the solvent was dissolved in 35 ml of ethyl acetate, and the stirring temperature was controlled between 20-25°C. After dissolution, 35 ml of n-hexane was added and stirred at 16-18°C for 1 hour. During this period, crystals should precipitate out. When a large amount of crystals precipitate out, add another 35 ml of n-hexane slowly within one hour, keeping the temperature between 16-18°C. After adding n-hexane, continue to stir the material for 2-3 hours, keeping the temperature at 15-16°C. Filter and wash the filter cake twice with 15 ml of cold n-hexane. The filter cake was ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com