Synthetic method for cordycepin
A synthesis method and technology of cordycepin, applied in chemical instruments and methods, preparation of sugar derivatives, sugar derivatives, etc., can solve the problems of toxicity, high price, and difficulty in pure products, and achieve mild reaction conditions, easy scale-up production, The effect of short reaction steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
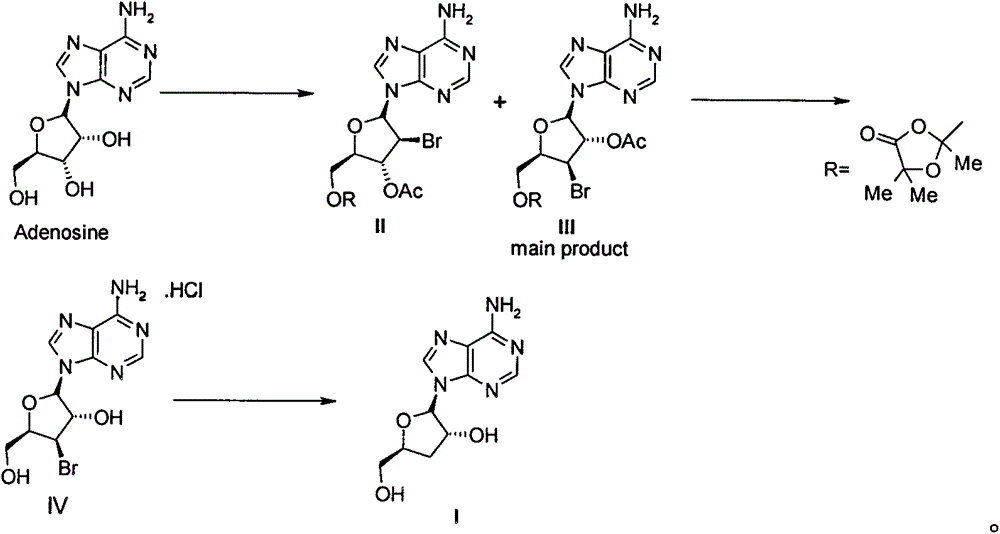
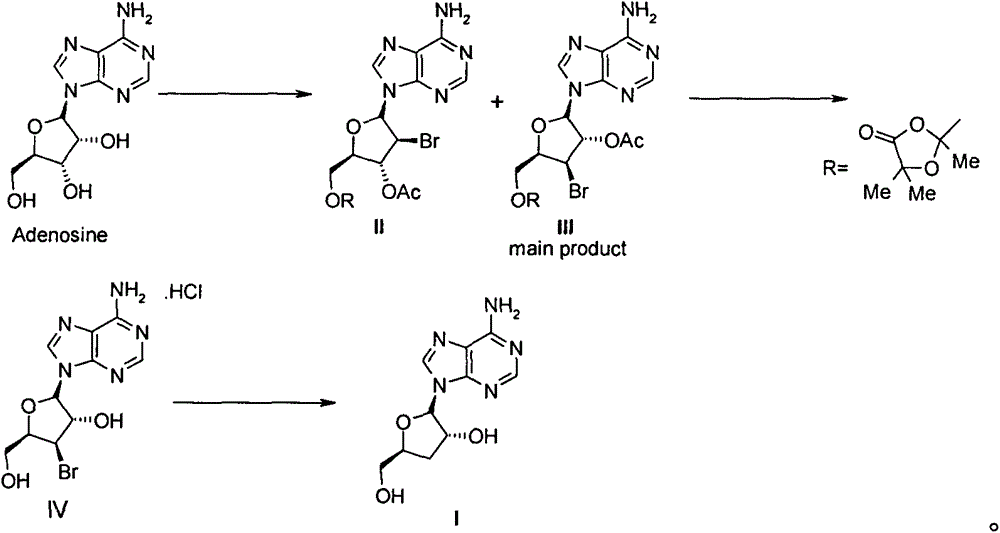
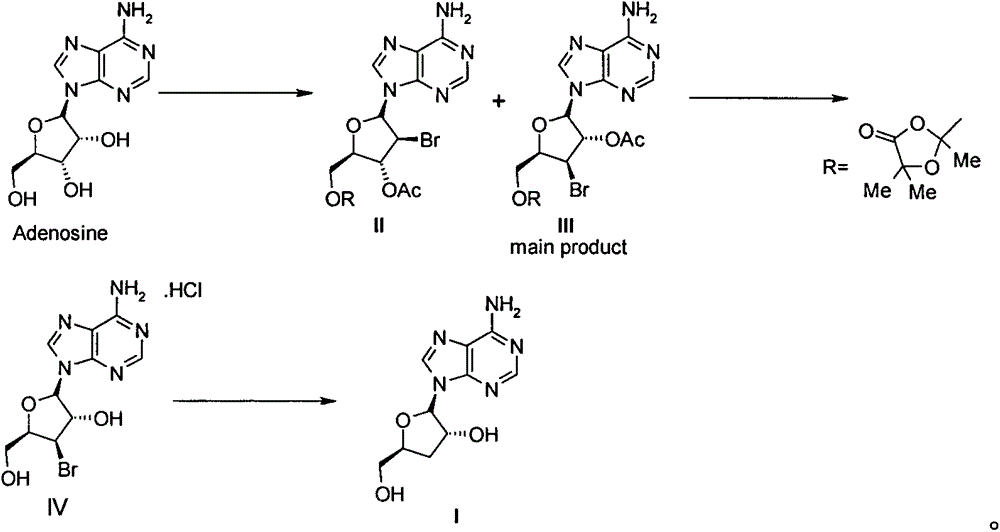
[0024] 1. 5'-[2,5,5-trimethyl-1,3-dioxolan-4-one-2-yl]-3'-bromo-3'-deoxy-2'-O- Acetyl adenosine (compound III) and 5'-[2,5,5-trimethyl-1,3-dioxolan-4-one-2-yl]-2'-bromo-2'- Preparation of Deoxy-3'-O-acetyladenosine (Compound II)
[0025] Adenosine (267g, 1mol) and ethyl acetate (2500mL) were added to a 5L three-necked flask, and then cooled in an ice-water bath to below 20°C, and 2-acetoxyisobutyryl (Mattock's Bromide, 580g, 420mL, 2.8 mol), keeping the temperature in the reaction solution below 25°C, the dropwise addition was completed in about 2 hours. After the dropwise addition was completed, the reaction was carried out at room temperature for 16 hours. Then pour the reaction solution into water / KHCO 3 (2L / 500g) in the solution prepared, stirred evenly, separated the organic layer, washed 3 times with 1L saturated brine, separated the organic phase, dried and concentrated to obtain 560g of white solid, namely 5'-[2,5,5- Trimethyl-1,3-dioxolan-4-one-2-yl]-3'-bromo-3'-d...
Embodiment 2
[0039] 1. Preparation of Compounds II and III
[0040] This step is identical with the step 1 of embodiment 1;
[0041] 2. Preparation of Compound IV
[0042] This step is identical with step 2 of embodiment 1;
[0043] 3, the preparation of cordycepin (compound I)
[0044] The compound IV (40g) obtained in step 2 was dissolved in ethanol (200mL) and water (200mL), then sodium acetate (40g) was added and stirred for 1 hour, then the insoluble matter was filtered off, and the obtained mother liquor was added to a 2L hydrogenation kettle , and then added 5% Pd / C (4g), vacuumed nitrogen replacement 3 times, and then hydrogenated at room temperature for 36 hours under the condition of 1-2atm. The catalyst was filtered off, washed twice with absolute ethanol, the combined mother liquor was concentrated under reduced pressure to about 100mL, and a large amount of solids were precipitated. After adding water (100mL) to completely dissolve the solids, add ethyl acetate (500L) and s...
Embodiment 3
[0046] 1. Preparation of Compounds II and III
[0047] This step is identical with the step 1 of embodiment 1;
[0048] 2. Preparation of Compound IV
[0049] This step is identical with step 2 of embodiment 1;
[0050] 3, the preparation of cordycepin (compound I)
[0051] The compound IV (40g) obtained in step 2 was dissolved in ethanol (200mL) and water (200mL), then sodium acetate (40g) was added and stirred for 1 hour, then the insoluble matter was filtered off, and the obtained mother liquor was added to a 2L hydrogenation kettle , and then add 10% Pd / C (2g), vacuum nitrogen replacement 3 times, and then hydrogenate at room temperature for 36 hours under the condition of 1-2atm. Filter out the catalyst, wash with absolute ethanol twice, combine the mother liquors, concentrate under reduced pressure to about 100mL, a large amount of solids precipitate, add water (100mL) to completely dissolve the solids, add ethyl acetate (500L) and stir for 15 minutes, separate the or...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com