Method for preparing high-entropy oxide material (MAlFeCuMg)3O4 by hydrothermal method
An oxide and hydrothermal technology, applied in the field of ceramic materials and infrared heating, can solve the problem that high-entropy oxide materials have not been retrieved, and achieve the effects of low cost, mild reaction conditions and high infrared emissivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
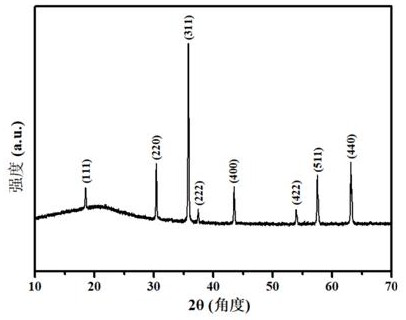
[0026] Embodiment 1, (CoAlFeCuMg) 3 o 4 Preparation and properties of high-entropy oxides
[0027] Weigh Co(NO 3 ) 2 ·6H 2 O 1.7484 g (0.006 mol), Al(NO 3 ) 3 9H 2 O 2.2588 g (0.006mol), Fe(NO 3 ) 3 9H 2 O 2.4283 g (0.006 mol), Cu(NO 3 ) 2 ·6H 2 O 1.4513 g (0.006 mol) and Mg(NO 3 ) 2 ·6H 2 O 1.5465 g (0.006 mol), were dissolved in 12 mL of ultrapure water and stirred evenly, and the five metal salt solutions were mixed and kept stirring until completely mixed to obtain a mixed solution of metal nitrate; then weighed 8.0701 g (0.024 mol ) of disodium edetate into the above mixed solution and stirred evenly; then the mixed solution was transferred to a polytetrafluoroethylene-lined stainless steel reactor and placed in a blast oven at 120°C for 1 h, then cooled to room temperature Finally, the reaction solution was filtered under reduced pressure and washed three times with ultrapure water, the precipitate was separated by suction filtration, and dried to obtain ...
Embodiment 2
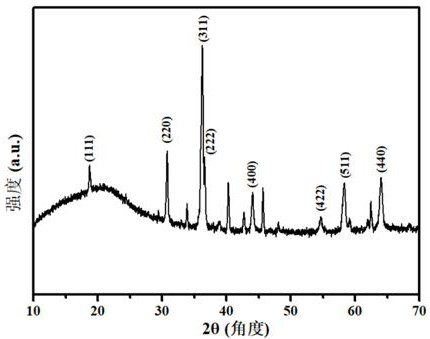
[0028] Embodiment 2, (CoAlFeCuMg) 3 o 4 Preparation and properties of high-entropy oxides
[0029] Weigh Co(NO 3 ) 2 ·6H 2 O 1.7484 g (0.006 mol), Al(NO 3 ) 3 9H 2 O 2.2588g (0.006mol), Fe(NO 3 ) 3 · 9H 2 O 2.4283g (0.006 mol), Cu(NO 3 ) 2 ·6H 2 O 1.4513 g (0.006 mol) and Mg(NO 3 ) 2 ·6H 2 O 1.5465 g (0.006 mol), respectively dissolved in 30 mL of ultrapure water and stirred evenly, and the five metal salt solutions were mixed and kept stirring until completely mixed to obtain a mixed solution of metal nitrate; then weighed 6.0517 g (0.018mol ) of disodium edetate) into the above mixed solution and stirred evenly; then the above mixed solution was transferred to a polytetrafluoroethylene-lined stainless steel reactor and placed in a blast oven at 120 °C for 1 h, cooled to After room temperature, the reaction solution was filtered under reduced pressure and washed three times with ultrapure water, the precipitate was separated by suction filtration, and dried to...
Embodiment 3
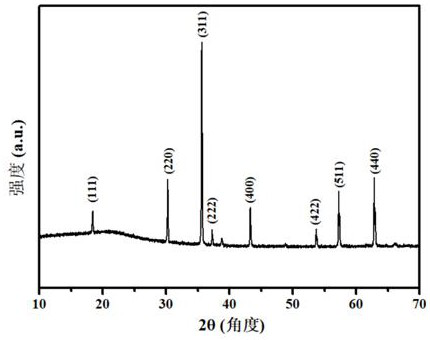
[0030] Embodiment 3, (CoAlFeCuMg) 3 o 4 Preparation and properties of high-entropy oxides
[0031] Weigh Co(NO 3 ) 2 ·6H 2 O 1.7484 g (0.006 mol), Al(NO 3 ) 3 9H 2 O 2.2588 g (0.006mol), Fe(NO 3 ) 3 · 9H 2 O 2.4283 g (0.006 mol), Cu(NO 3 ) 2 ·6H 2 O 1.4513 g (0.006 mol) and Mg(NO 3 ) 2 ·6H 2 O 1.5465 g (0.006 mol), respectively dissolved in 10 mL of ultrapure water and stirred evenly, and the five metal salt solutions were mixed and kept stirring until completely mixed to obtain a mixed solution of metal nitrate; then weighed 10.0862 g (0.030 mol ) of disodium edetate into the above mixed solution and stirred evenly; then the mixed solution was transferred to a polytetrafluoroethylene-lined stainless steel reactor and placed in a blast oven at 120°C for 1 h, then cooled to room temperature Finally, the reaction solution was filtered under reduced pressure and washed 7 times with ultrapure water, the precipitate was separated by suction filtration, and dried to ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| emissivity | aaaaa | aaaaa |
| emissivity | aaaaa | aaaaa |
| emissivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com