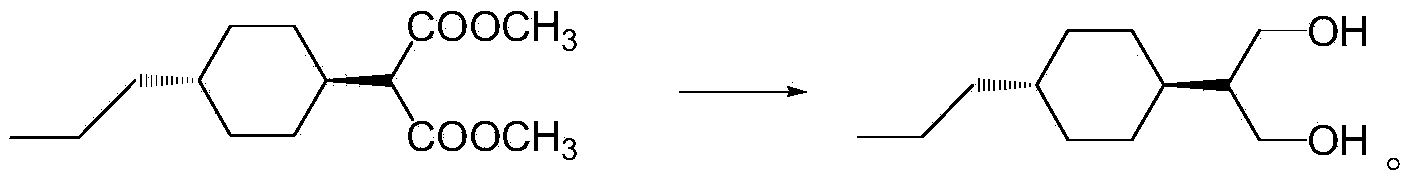Preparation method of 1,3-propanediol derivatives and intermediates
A technology of propylene glycol and derivatives, which is applied in the field of preparation of 1,3-propanediol derivatives and intermediates, and can solve problems such as high cost, complicated process, and environmental protection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
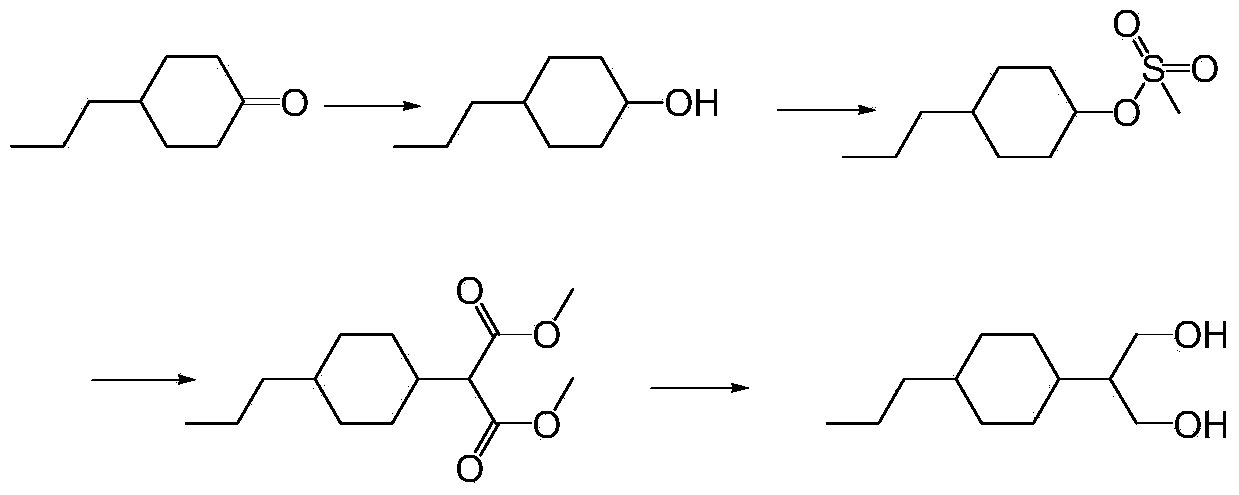
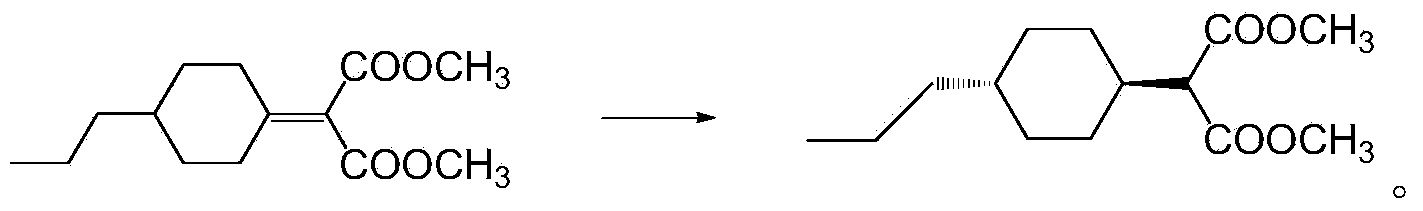
[0079] Preparation of dimethyl 2-(4-n-propylcyclohexylene)-1,3-malonate
[0080] Under the protection of nitrogen, add 284g (1.5mol) titanium tetrachloride and 1425g dichloromethane to the 2L reaction flask, cool down, add dropwise 84g (0.6mol) propyl cyclohexanone and A mixed solution of 95g (0.72mol) dimethyl malonate and 285g THF, after dropping, keep warm for 1h, add 220g (3mol) diethylamine dropwise at -5°C ~ 0°C, after dropwise, keep warm for 2h, then Raise the temperature to 5°C, keep the heat for another 4 hours, and control the reaction until GC (propyl cyclohexanone) < 0.5%. After passing the test, transfer the reaction solution to a 5L beaker, add 500g of water to wash, let stand to separate the liquid, and use 50g of di Chloromethane stripped the water layer again, combined the organic layers, evaporated about 1442g of solvent under reduced pressure, cooled the system to 25°C, added 300g of petroleum ether for extraction, washed twice with 250g of 5% NaCl solution,...
Embodiment 2
[0082] Preparation of dimethyl 2-(4-n-propylcyclohexylene)-1,3-malonate
[0083] Under the protection of nitrogen, add 284g (1.5mol) titanium tetrachloride and 1425g dichloromethane to the 2L reaction flask, cool down, add dropwise 84g (0.6mol) propyl cyclohexanone and A mixed solution of 95g (0.72mol) dimethyl malonate and 285g THF, after dropping, keep warm for 1h, add 93.3g (3mol) methylamine dropwise at -5°C ~ 0°C, after dropwise, keep warm for 2h, then Raise the temperature to 5°C, keep the heat for another 4 hours, and control the reaction until GC (propyl cyclohexanone) < 0.5%. After passing the test, transfer the reaction solution to a 5L beaker, add 500g of water to wash, let stand to separate the liquid, and use 50g of di Chloromethane stripped the water layer again, combined the organic layers, evaporated about 1442g of solvent under reduced pressure, cooled the system to 25°C, added 300g of petroleum ether for extraction, washed twice with 250g of 5% NaCl solution,...
Embodiment 3
[0085] Preparation of dimethyl 2-(4-n-propylcyclohexylene)-1,3-malonate
[0086]Under the protection of nitrogen, add 284g (1.5mol) titanium tetrachloride and 1425g dichloromethane to the 2L reaction flask, cool down, add dropwise 84g (0.6mol) propyl cyclohexanone and A mixed solution of 95g (0.72mol) dimethyl malonate and 285g THF, after dropping, keep warm for 1h, add 135.2g (3mol) dimethylamine dropwise at -5℃~0℃, after dropping, keep warm for 2h, Then raise the temperature to 5°C, keep the temperature for another 4 hours, and control the reaction until GC (propyl cyclohexanone) <0.5%. After passing the test, transfer the reaction solution to a 5L beaker, add 500g of water to wash, let stand to separate the liquid, and use 50g The dichloromethane back-extracted the water layer once more, combined the organic layers, evaporated about 1442g of solvent under reduced pressure, cooled the system to 25°C, added 300g of petroleum ether for extraction, washed twice with 250g of 5% ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com