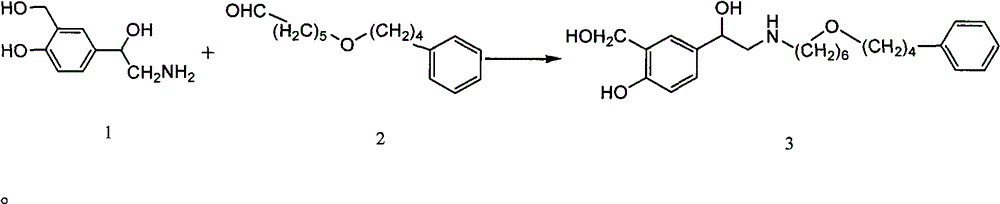
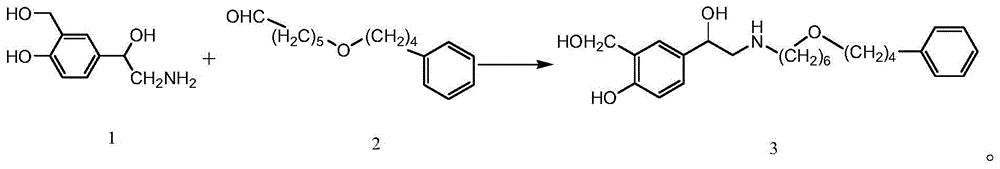
Preparation method for salmeterol
A technology of palladium carbon and compounds, applied in the field of preparation of salmeterol, can solve the problems of low yield and difficulty in applying industrial production, etc., and achieve the effects of high yield, low cost, and simple operation process and post-treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Add 0.4g (2.2mmol) of compound 1 and 0.5g (2mmol) of compound 2 to the autoclave successively, 0.05g mass fraction is 10%Pd / C, 50ml absolute ethanol, seal, pass into 2.0Mpa hydrogen, room temperature Stir for 12h. Filter, concentrate the filtrate, wash with 50ml of ethyl acetate, filter, adjust the pH of the filtrate to 2-3 with 2mol / L hydrochloric acid, separate layers, extract the ethyl acetate layer with water, combine the water layers, and adjust with saturated sodium bicarbonate solution The pH of the aqueous layer was 8-9, extracted with ethyl acetate, combined the ethyl acetate layers, washed with water, washed with saturated brine, dried over anhydrous sodium sulfate, filtered, and the filtrate was concentrated to obtain an oil, which was stirred by adding 10ml of methyl tert-butyl ether 0.67 g of white solid salmeterol was precipitated, with a yield of 80.7%. The structural identification data of the product are as follows:
[0028] m.p.75-76°C (75-77°C repor...
Embodiment 2
[0033] Add 0.46g (2.5mmol) of compound 1 and 0.5g (2mmol) of compound 2 to the autoclave successively, 0.1g of mass fraction is 20%Pd / C, 50ml of absolute ethanol, seal it, pass into 2.0Mpa hydrogen, room temperature Stir for 24h. Filter, concentrate the filtrate, wash with 50ml of ethyl acetate, filter, adjust the pH of the filtrate to 2-3 with 2mol / L hydrochloric acid, separate layers, extract the ethyl acetate layer with water, combine the water layers, and adjust with saturated sodium bicarbonate solution The pH of the aqueous layer was 8-9, extracted with ethyl acetate, combined the ethyl acetate layers, washed with water, washed with saturated brine, dried over anhydrous sodium sulfate, filtered, and the filtrate was concentrated to obtain an oil, which was stirred by adding 10ml of methyl tert-butyl ether , 0.68 g of salmeterol was precipitated as a white solid, with a yield of 81.9%. The structural identification data of the product are as follows:
[0034] m.p.76-77°...
Embodiment 3
[0039] Add 0.4g (2.2mmol) of compound 1 and 0.82g (3.3mmol) of compound 2 successively in the autoclave, the mass fraction of 0.04g is 10% Pd / C, 50ml of absolute ethanol, seal, and feed 2.0Mpa hydrogen, Stir at room temperature for 12h. Filter, concentrate the filtrate, wash with 50ml of ethyl acetate, filter, adjust the pH of the filtrate to 2-3 with 2mol / L hydrochloric acid, separate layers, extract the ethyl acetate layer with water, combine the water layers, and adjust with saturated sodium bicarbonate solution The pH of the aqueous layer was 8-9, extracted with ethyl acetate, combined the ethyl acetate layers, washed with water, washed with saturated brine, dried over anhydrous sodium sulfate, filtered, and the filtrate was concentrated to obtain an oil, which was stirred by adding 10ml of methyl tert-butyl ether , 0.67 g of salmeterol was precipitated as a white solid, with a yield of 73.3%. The structural identification data of the product are as follows:
[0040] m.p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com