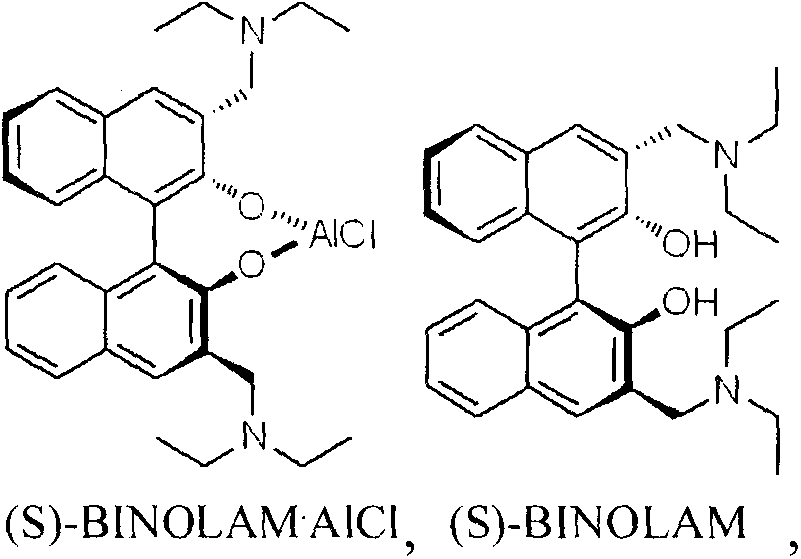
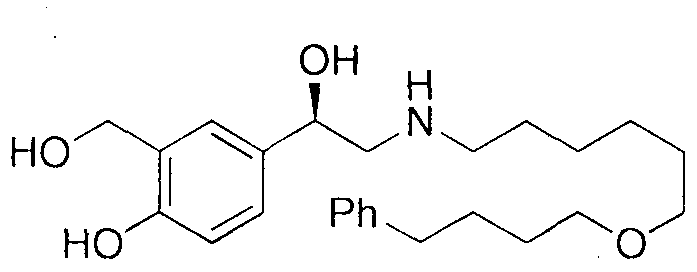
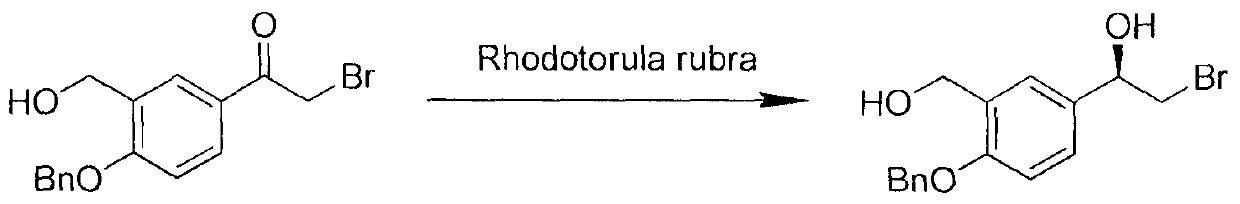
Method for synthesizing (R)-salmeterol
A compound and molar ratio technology, applied in the field of drug synthesis, can solve the problems of non-recyclability, serious and highly toxic oxazaborolane environmental pollution, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1: 4-Hydroxy-3-Hydroxymethylbenzaldehyde 2 Synthesis
[0038] 12.20 g (0.10 mol) of p-hydroxybenzaldehyde, 40% hydrochloric acid (88 mL), and 6 g (0.20 mol) of paraformaldehyde were successively added to a 250 mL four-necked flask, and stirred at 60° C. for 3 h. Cool to room temperature, filter to obtain a white solid, wash with water (300mL×3), filter with suction, dry to obtain a white solid, wash with an appropriate amount of methanol to obtain a white solid compound 2 , yield 63%, melting point 142-143 °C, 1 H-NMR: (400MHz, DMSO-d 6 )δ9.81(s, 1H, CHO), 7.89(s, 1H, Ar-H), 7.65(d, J=8.0Hz, 1H, Ar-H), 6.94(d, J=8.0Hz, 1H, Ar-H) 5.17(s, 1H, OH), 4.51(s, 2H, CH 2 ).
Embodiment 2
[0039] Example 2: 4-Hydroxy-3-Hydroxymethylbenzaldehyde 2 Synthesis
[0040] Add 12.20g (0.10mol) of p-hydroxybenzaldehyde, dilute sulfuric acid (100mL) with a mass fraction of 20%, and 6g (0.20mol) of paraformaldehyde into a 250mL four-necked flask in sequence, stir at 50°C and pass in hydrogen chloride gas 4h. Cool to room temperature, filter to obtain a white solid, wash with water (300mL×3), filter with suction, dry to obtain a white solid, wash with an appropriate amount of methanol to obtain a white solid compound 2 , yield 51%.
Embodiment 3
[0041] Example 3: 4-Hydroxy-3-Hydroxymethylbenzaldehyde 2 Synthesis
[0042] Add 12.20 g (0.10 mol) of p-hydroxybenzaldehyde, boric acid (80 mL), and 6 g (0.20 mol) of paraformaldehyde into a 250 mL four-necked flask in sequence, stir at 65° C. and pass in hydrogen chloride gas for 2 h. Cool to room temperature, filter to obtain a white solid, wash with water (300mL×3), filter with suction, dry to obtain a white solid, wash with an appropriate amount of methanol to obtain a white solid compound 2 , yield 63%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com