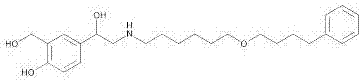
Method for preparing salmeterol transdermal patch
A technology of transdermal patch and penetration enhancer, applied in the field of prevention and treatment of asthma, salmeterol transdermal patch, can solve the problems of complex preparation process and drug delivery device
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
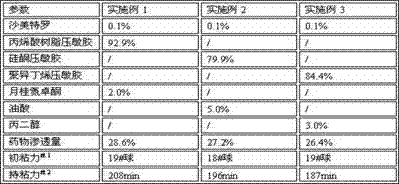
[0018] Weigh 1.0 g of salmeterol, add 50.0 g of ethyl acetate to dissolve, add 20.0 g of laurocapram, stir and mix, then add 929.0 g of acrylic pressure-sensitive adhesive solution, mix and stir for 1 hour, and use a vacuum pump to remove air bubbles to obtain a uniform Colloidal solution, put on the coating machine, adjust the coating thickness to 0.1mm, coat on the backing layer, dry at 60°C for 2 hours, press with PET polyester film, divide into 2cm×2cm size (0.1mg / paste) , ready to pack. The resulting patch was applied to the back of depilated mice for 24 hours. Percent drug permeability was calculated by high performance liquid chromatography measuring the amount of drug in the patch before and 24 hours after application of the salmeterol patch.
Embodiment 2
[0020] Weigh 1.0 g of salmeterol, add 150.0 g of petroleum ether to dissolve, then add 50.0 g of oleic acid, stir and mix, add 799.0 g of silicone pressure-sensitive adhesive, mix and stir for 1 hour, and vacuum pump to remove air bubbles to obtain a uniform colloidal solution. Put it on the coating machine, adjust the coating thickness to 0.1mm, coat it on the backing layer, dry it at 60°C for 2 hours, press it with PET polyester film, divide it into 2cm×2cm size (0.1mg / paste), and pack it. . The resulting patch was applied to the back of depilated mice for 24 hours. Percent drug permeability was calculated by high performance liquid chromatography measuring the amount of drug in the patch before and 24 hours after application of the salmeterol patch.
Embodiment 3
[0022] Weigh 1.0 g of salmeterol, add 125.0 g of dichloromethane to dissolve, add 30.0 g of propylene glycol, stir and mix, add 844.0 g of polyisobutylene pressure-sensitive adhesive, mix and stir for 1 hour, and vacuum pump to remove air bubbles to obtain a uniform colloidal solution , on the coating machine, adjust the coating thickness to 0.1mm, coat on the backing layer, dry at 60°C for 2 hours, press it with PET polyester film, divide it into 2cm×2cm (0.1mg / paste), pack it have to. The resulting patch was applied to the back of depilated mice for 24 hours. Percent drug permeability was calculated by high performance liquid chromatography measuring the amount of drug in the patch before and 24 hours after application of the salmeterol patch.
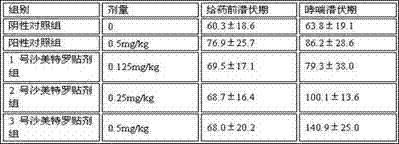
[0023] The drug permeability and other parameters of Example 1, Example 2, and Example 3 were compared. The types and contents of pressure-sensitive adhesives, solvents, and penetration enhancers used in different examples were diff...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com