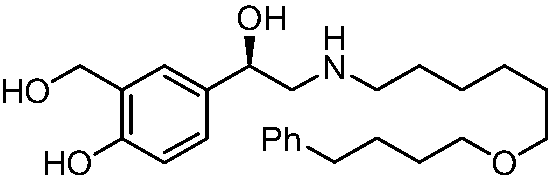
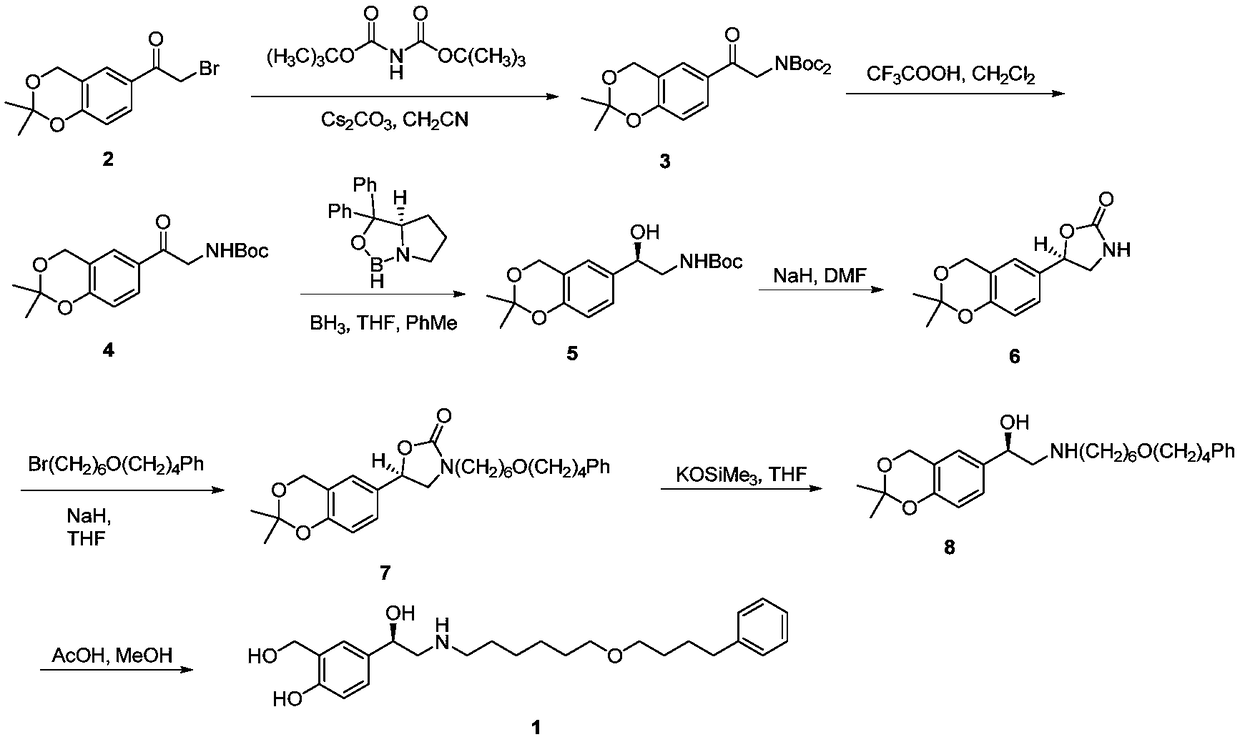
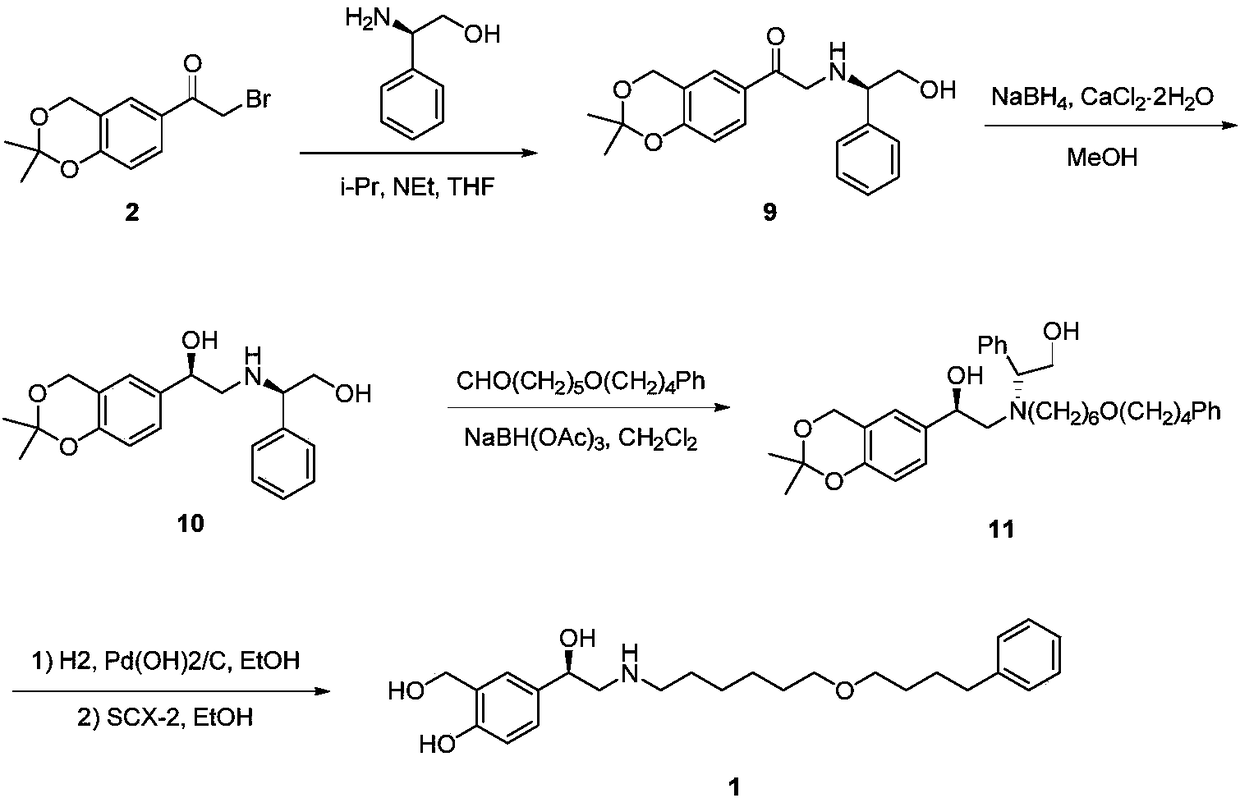
A kind of synthetic method of r-salmeterol
A synthesis method and compound technology, applied in the field of synthesis of drug R-salmeterol, can solve the problems of unstable product quality, poor stereoselectivity, harsh reaction conditions, etc., and achieve low production cost, simple route, and mild reaction conditions Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Embodiment 1: the preparation of compound (II)
[0057] Weigh bromide 10g (30.5mmol), dissolve in 80mL dichloromethane, add K 2 CO 3 5.0 g (36.6 mmol), and 12.4 g (36.6 mmol) of N-benzyl-6-(4-phenylbutoxy)-1-hexylamine were slowly added dropwise under stirring, and the reaction system gradually became cloudy. React at room temperature (25°C) for 5 hours, stop stirring, remove the solid in the reaction liquid by suction filtration, add ethyl acetate (100 mL) to the filtrate to dilute, then wash with water and saturated brine in sequence, after the pH detection of the organic phase is neutral, anhydrous Dry over sodium sulfate, filter, and remove the solvent by rotary evaporation to obtain 17 g of oily product (II), with a yield of 95%.
[0058] 1 H NMR (CDCl 3 ):δ1.25-1.28(m,3H),1.50-1.67(m,9H),2.07(s,3H),2.34(s,3H),2.58-2.65(m,4H),3.32-3.41(m ,6H),3.72(s,1H),3.80(s,1H),5.07(s,2H),7.14-7.19(m,6H),7.24-7.34(m,7H).
Embodiment 2
[0059] Embodiment 2: the preparation of compound (II)
[0060] Weigh 10g (30.5mmol) of the bromide, dissolve it in 80mL of dichloromethane, add 3.7g (36.6mmol) of triethylamine, slowly add N-benzyl-6-(4-phenylbutoxy)- 12.4 g (36.6 mmol) of 1-hexylamine, the reaction system gradually became cloudy. React at room temperature (25°C) for 5 hours, stop stirring, remove the solid in the reaction liquid by suction filtration, add ethyl acetate (100 mL) to the filtrate to dilute, then wash with water and saturated brine in sequence, after the pH detection of the organic phase is neutral, anhydrous After drying over sodium sulfate, the solvent was removed by rotary evaporation to obtain 16 g of yellow oily product (II), with a yield of 90%.
[0061] 1 H NMR (CDCl 3 ):δ1.25-1.28(m,3H),1.50-1.67(m,9H),2.07(s,3H),2.34(s,3H),2.58-2.65(m,4H),3.32-3.41(m ,6H),3.72(s,1H),3.80(s,1H),5.07(s,2H),7.14-7.19(m,6H),7.24-7.34(m,7H).
Embodiment 3
[0062] Embodiment 3: the preparation of compound (II)
[0063] Weigh 10g (30.5mmol) of bromide, dissolve it in 60mL of methyl isobutyl ketone, add 3.7g (36.6mmol) of triethylamine, slowly add N-benzyl-6-(4-phenylbutoxy Base)-1-hexylamine 12.4g (36.6mmol), the reaction system gradually became cloudy. React at room temperature (25°C) for 5 hours, stop stirring, remove the solid in the reaction liquid by suction filtration, add ethyl acetate (100 mL) to the filtrate to dilute, then wash with water and saturated brine in sequence, after the pH detection of the organic phase is neutral, anhydrous Dry over sodium sulfate, filter, and remove the solvent by rotary evaporation to obtain 16.5 g of yellow oily product (II), with a yield of 92%.
[0064] 1 H NMR (CDCl 3 ):δ1.25-1.28(m,3H),1.50-1.67(m,9H),2.07(s,3H),2.34(s,3H),2.58-2.65(m,4H),3.32-3.41(m ,6H),3.72(s,1H),3.80(s,1H),5.07(s,2H),7.14-7.19(m,6H),7.24-7.34(m,7H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com