Formoterol superfine formulation
a technology of formoterol and superfine, applied in the direction of drug compositions, dispersed delivery, aerosol delivery, etc., can solve the problems of mucosal damage, fibrosis of lung tissue, and irreversible narrowing of airways and lung tissue,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Superfine Formoterol HFA Formulation
[0090] A formulation was prepared with the composition as follows:
AmountsPer unitNominal doseComponents%μgFormoterol fumarate1.92mg0.019w / v12Anhydrous ethanol1416.7mg12w / w—HCl 1 M4.40mg*0.037w / w—HFA 134a (q.s.11808mg——to 10.09 ml)
*equivalent to 4.35 μl
[0091] The formulation (120 actuations / canister, overage of 40 actuations) was filled in standard aluminum canisters (two stage pressure filling) under pressure and fitted with a metering valve having a 63 μl metering chamber. Two actuators were used with orifice diameter of 0.30 and 0.42 mm. Results were obtained as a mean of 2 cans.
[0092] The aerodynamic particle size distribution was determined by ACI, according to the description on page 17 lines 4 to 12.
[0093] The delivery characteristics of the formulation are reported in Table 1 in comparison with the reference CFC formulation currently available on the market (Foradil). In particular the following parameters are reported: i) nominal dos...
example 2
Pharmacokinetics Study
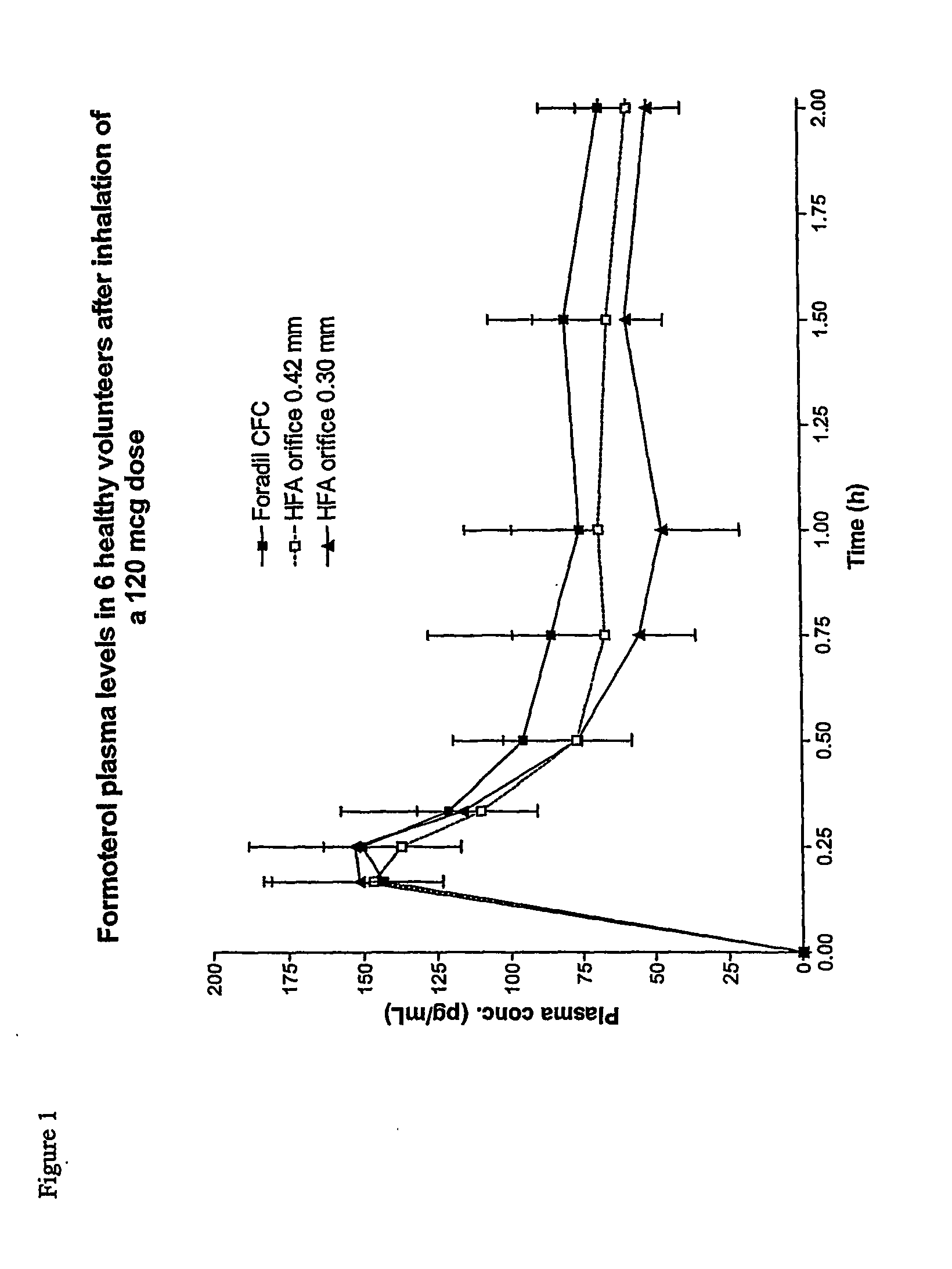
[0095] The aim of the study was to evaluate the pharmacokinetics of formoterol in 6 healthy volunteers after single administration of the formoterol formulations of Example 1 at 120 μg dose (10 shots×12 μg / shot) in comparison with the marketed CFC formulation (Foradil). The experimental protocol is reported as follows:
[0096] Treatments [0097] Foradil CFC 120 μg. (10 shots×12 μg / shot): Reference formulation [0098] Formoterol / HFA orifice 0.42 mm 120 μg. (10 shots×12 μg / shot): Test formulation [0099] Formoterol / HFA orifice 0.30 mm 120 μg. (10 shots×12 μg / shot): Test formulation
[0100] The study was a single dose cross-over study; subjects received the drug at 8 a.m. The wash-out among different treatments was of at least 1 weeks. Patients were instructed to take 10 doses. Time 0 for each dose was defined as the time when the MDI is first actuated.
[0101] Bioanalysis
[0102] Assay of formoterol was carried out employing HPLC / MS validated method with a LOQ of 2 pg / ...
example 3
Effect of the Residual Humidity on the Formoterol Assay
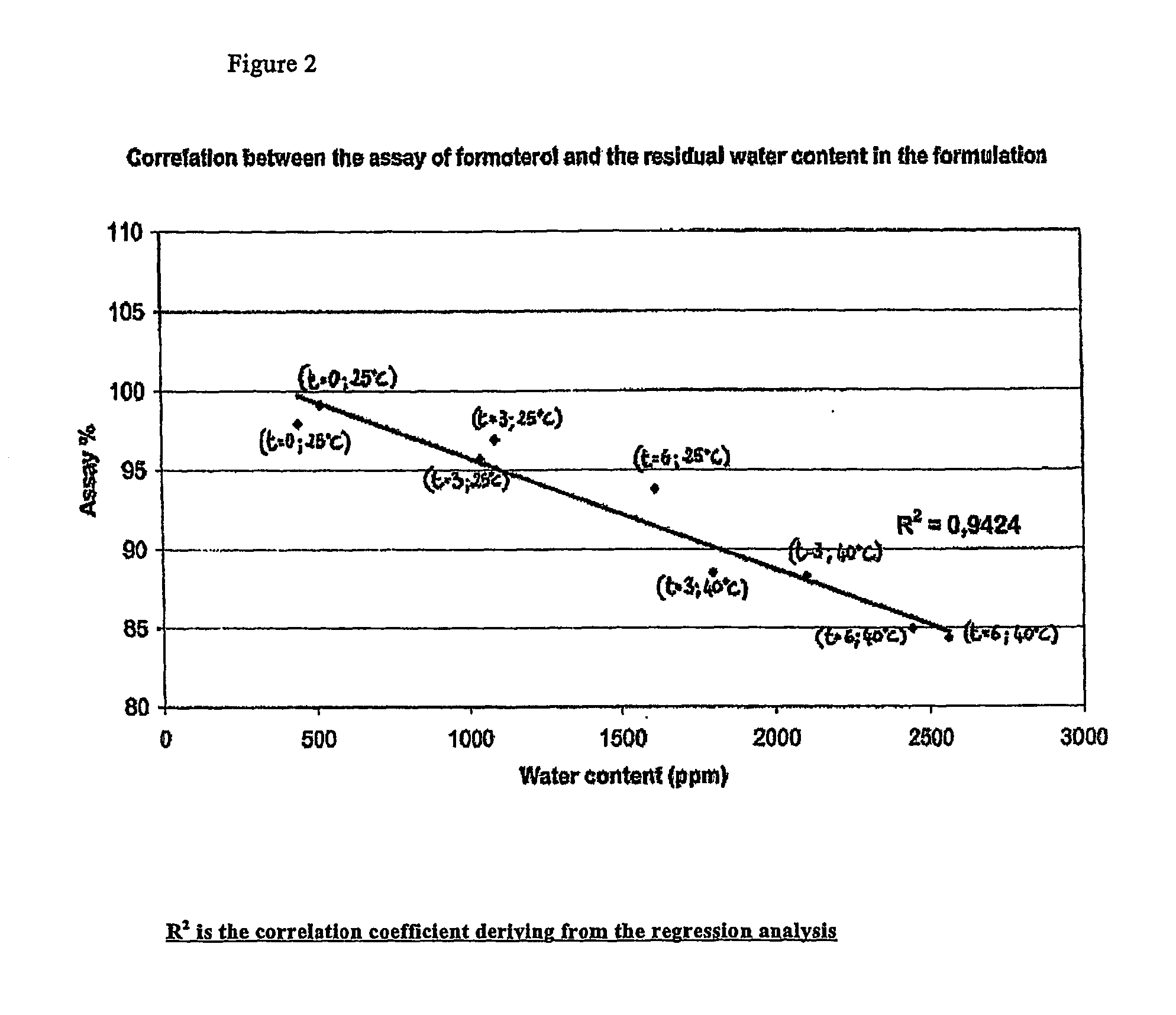
[0110] The formulation of Example 1 filled in standard aluminum cans was stored in different conditions (25° C., 40° C.) and for different times (0, 3, 6 months).
[0111] The assay of formoterol was determined by HPLC while the water content was determined by Karl-Fischer method.
[0112] The results, reported in FIG. 2, show an inverse linear correlation between the assay of formoterol and the residual amount of water. The numbers between brackets refer to time and temperature condition, respectively. The formoterol assay for a residual humidity lower than 1500 ppm meets the requirements of the ICH guideline Q1A, whereas for a residual humidity higher than 1500 ppm, the assay decreases below 90%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| aerodynamic diameters | aaaaa | aaaaa |
| diameters | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com