Budesonide and formoterol spray inhalation suspension and preparation method thereof
A technology of atomization inhalation and budesonide, which is applied in the directions of pharmaceutical formulation, liquid delivery, dispersion liquid delivery, etc., can solve the problems of inaccurate delivered dose, low compliance, usage restrictions, etc., and achieve accurate, controllable and compliant delivery dose. The effect of good sex and avoiding potential harm
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
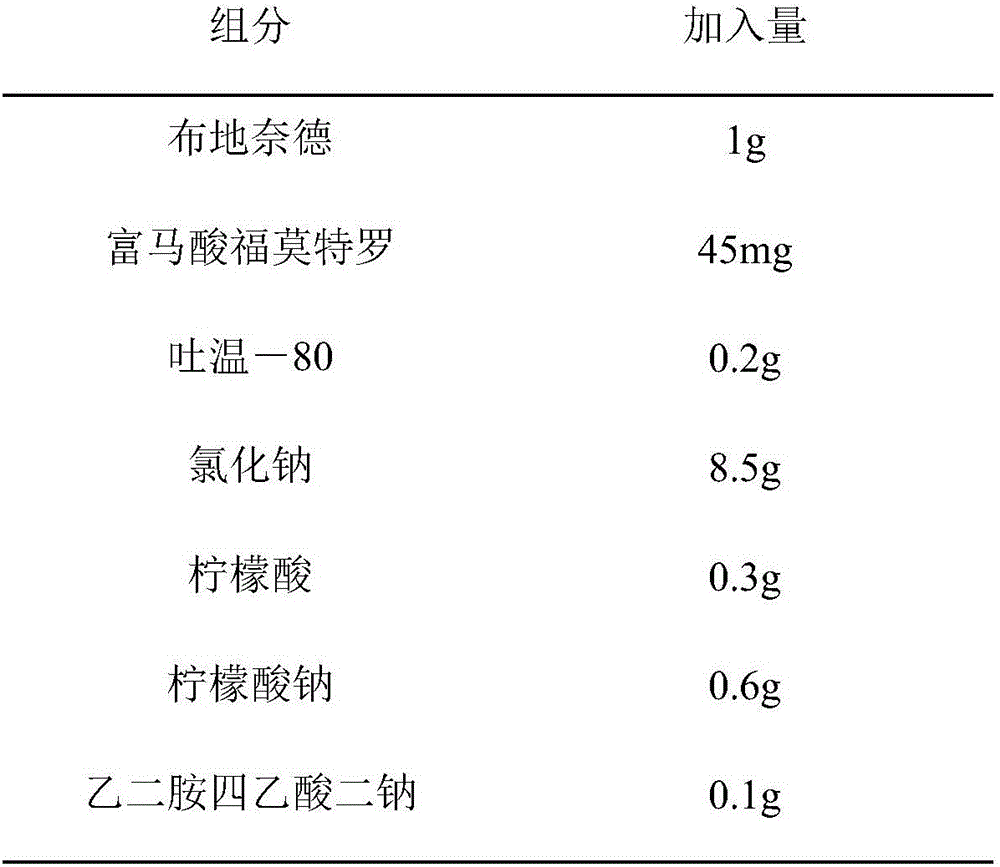
[0034]
[0035] Add water to 1000ml, adjust pH to 5.00 with sodium hydroxide
[0036] Step A: dissolving formoterol fumarate in water to form solution a, adding sodium chloride, disodium edetate, citric acid and sodium citrate to solution a, and ultrasonically dispersing and dissolving to form solution b;
[0037] Step B: filter and sterilize solution b with a 0.22 μm membrane filter;
[0038] Step C: Dissolving Tween-80 in water to obtain solution c, adding budesonide to solution c, performing ultrasonic dispersion, then performing high-pressure homogenization treatment at a pressure of 500 bar, and circulating 5 times to obtain a suspension d;
[0039] Step D: Sterilize the suspension d at 120°C for 15 minutes;
[0040] Step E: Mix solution b and suspension d evenly under aseptic conditions, add water for injection to 1000ml, adjust pH to 5.0 with sodium hydroxide, and aseptically dispense them into single-dose packaging containers.
Embodiment 2
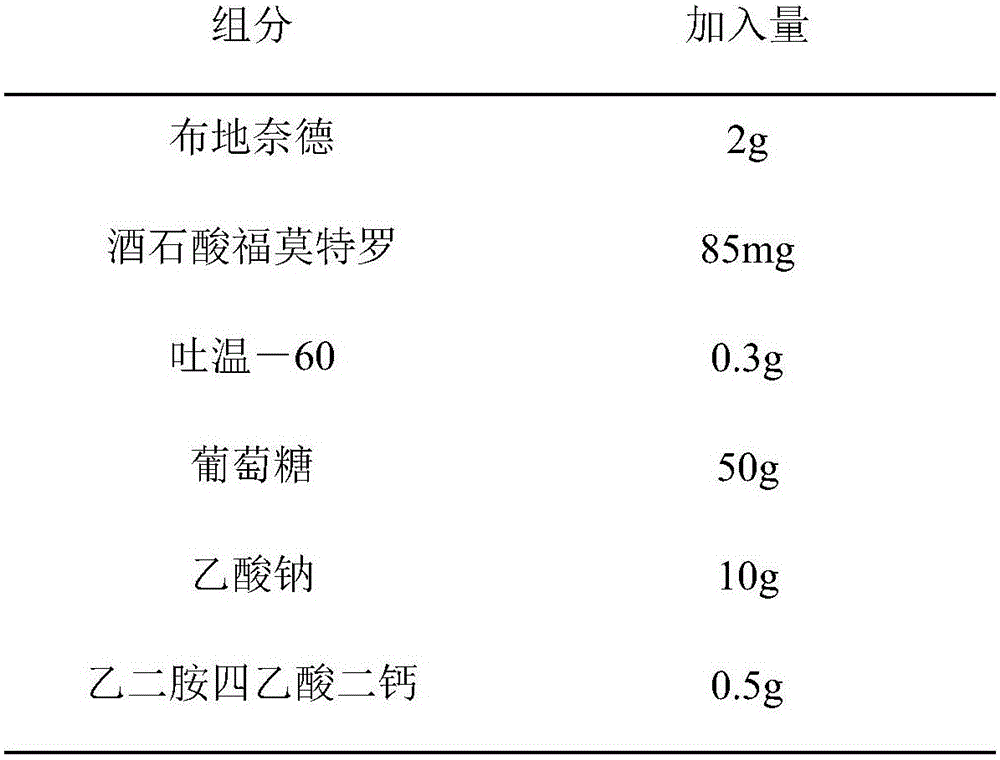
[0042]
[0043] Add water to 1000ml, adjust pH to 4.00 with acetic acid
[0044] Preparation:
[0045] Step A: dissolving formoterol tartrate in water to form solution a, adding sodium chloride, sodium acetate and dicalcium diamine tetraacetate to solution a, and ultrasonically dispersing and dissolving to form solution b;
[0046] Step B: filter and sterilize solution b with a 0.22 μm membrane filter;
[0047] Step C: dissolving Tween-60 in water to obtain solution c, adding budesonide to solution c, performing ultrasonic dispersion, then performing high-pressure homogenization treatment at a pressure of 1500 bar, and circulating 3 times to obtain a suspension d;
[0048] Step D: Sterilize the suspension d at 115°C for 30 minutes;
[0049] Step E: Mix solution b and suspension d evenly under aseptic conditions, add water for injection to 1000ml, adjust pH to 4.0 with acetic acid, and aseptically dispense them into single-dose packaging containers.
Embodiment 3
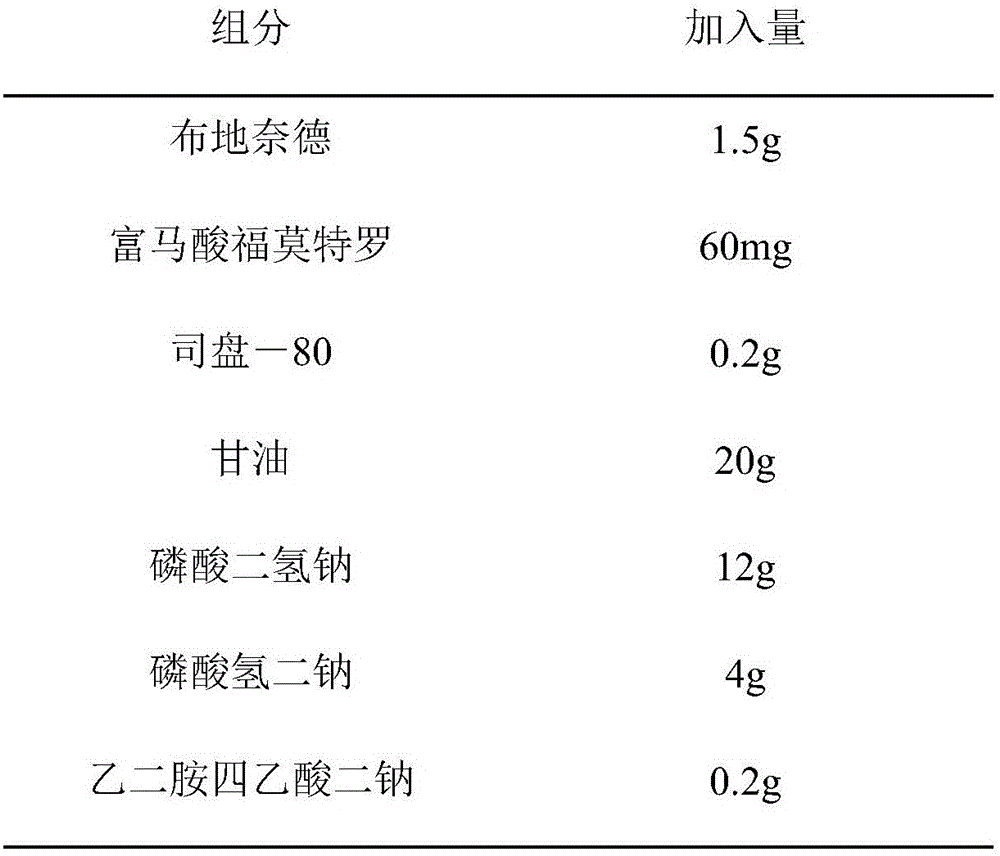
[0051]
[0052] Add water to 1000ml and adjust the pH to 6.00 with sodium hydroxide.
[0053] Preparation:
[0054] Step A: Dissolve formoterol fumarate in water to form solution a, add glycerin, sodium dihydrogen phosphate, disodium hydrogen phosphate and disodium edetate to solution a, and ultrasonically disperse and dissolve to form solution b ;
[0055] Step B: filter and sterilize solution b with a 0.22 μm membrane filter;
[0056] Step C: Dissolve Span-80 in water to obtain solution c, add budesonide to solution c, perform ultrasonic dispersion, then perform high-pressure homogenization treatment at a pressure of 1000 bar, and circulate 4 times to obtain suspension d ;
[0057] Step D: Sterilize the suspension d at 120°C for 20 minutes;
[0058] Step E: Mix solution b and suspension d evenly under aseptic conditions, add water to 1000ml, and adjust the pH to 6.0 with sodium hydroxide.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com