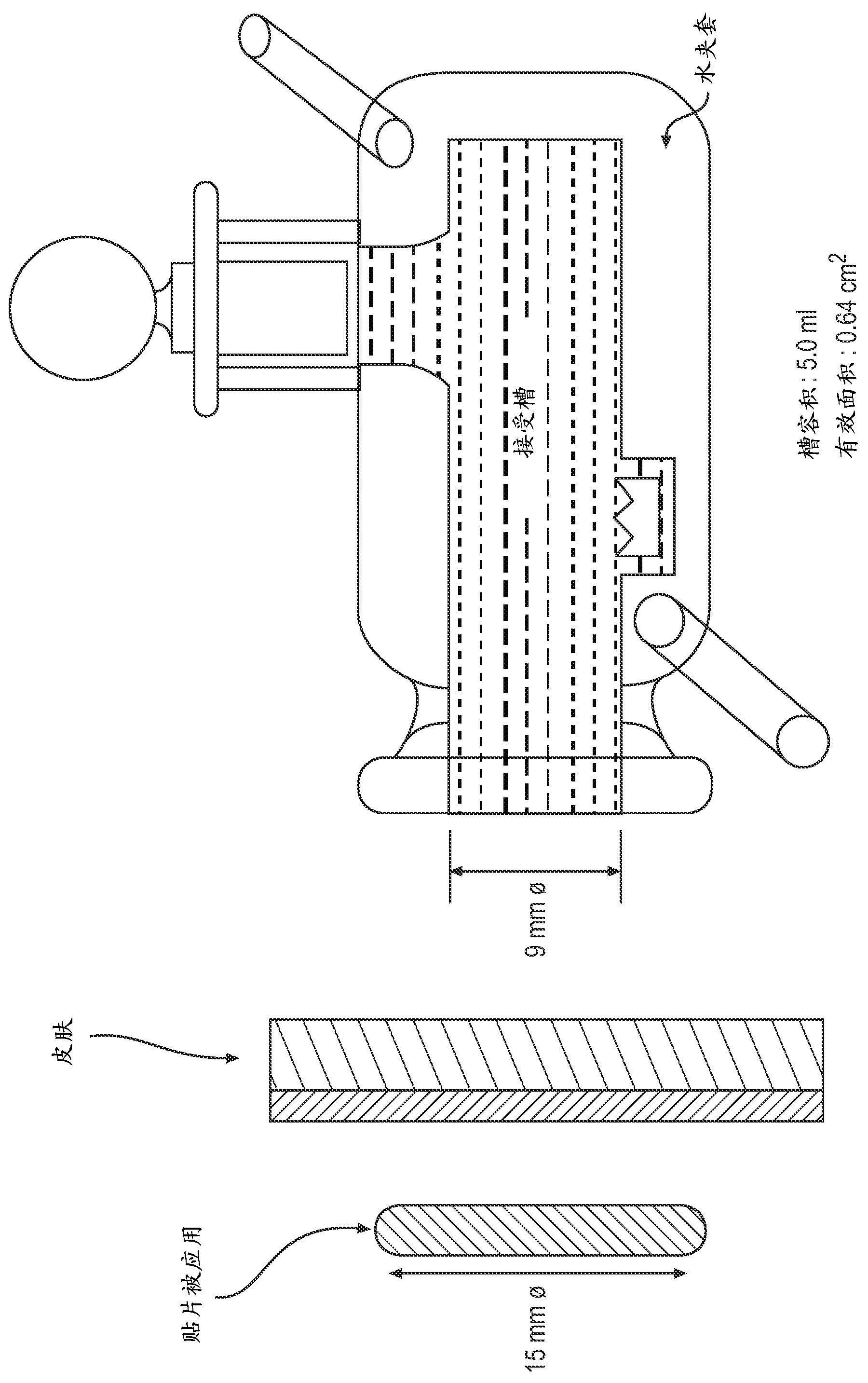
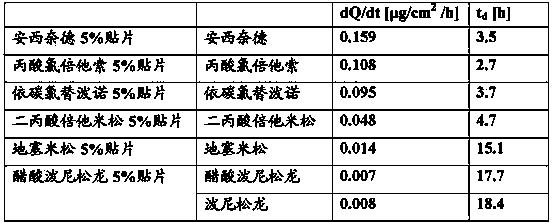
Transdermal drug delivery system and method of using the same
A transdermal drug delivery system and skin technology, applied in the field of eyelid disease treatment, can solve the problems of impairing the quality of life of patients, unsightly, and not mentioned
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
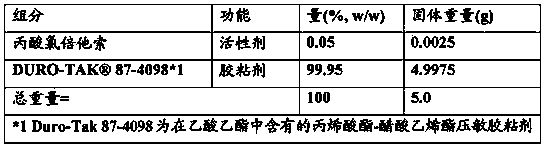
[0081] By blending 100 parts by weight of hydrogenated rosin ester resin (trade name "Pinecrystal KE311") as a tackifier with 100 parts by weight of styrene-isoprene-styrene block copolymer (trade name " Quintac 3520"), resulting in SIS (Styrene-Isoprene-Styrene) based pressure sensitive adhesives. Dissolve 99.0 w / w% SIS-based pressure-sensitive adhesive (Pinecrystal KE311 / Quintac 3520 ratio 50% / 50% (w / w)) and 1.0 w / w% clobetasol propionate in toluene to A coating liquid having a solids content of 50% by weight was obtained. The coating liquid was applied on a release paper so as to obtain a dry coating thickness of 40 µm. After drying, a support (a polyester film having a thickness of 12 μm) was laminated to provide a patch preparation.
Embodiment 2
[0083] Add 0.005 g of a crosslinking agent (chelate type crosslinking agent; trade name "NISSETSU CK-401") and 0.05 g of clobetasol to 12.363 g (solid: 4.945 g) of an acrylic pressure-sensitive adhesive [trade name "NISSETSU PE-300"; alkyl (meth)acrylate-vinyl acetate copolymer; pressure-sensitive adhesive solution (ethyl acetate / toluene mixed solvent) with a solid content of 40% by weight] to prepare The coating liquid had a concentration of 57.3% by weight. The coating liquid was applied on a release paper so as to obtain a dry coating thickness of 80 µm. After drying, a support (a polyester film having a thickness of 12 μm) was laminated to provide a patch preparation.
Embodiment 3
[0085] By using 40.5 g of styrene-isoprene-styrene block copolymer (trade name "SIS5000") as a rubber elastic substance and 40.5 g of terpene resin (trade name "YS Resin 1150N") as a tackifier and 10 g of clobetasol propionate were dissolved in 150 g of toluene to obtain a pressure-sensitive adhesive solution (coating liquid) having a solid content of 40% by weight. The coating liquid was applied on a release paper so as to obtain a dry coating thickness of 40 µm. After drying, a support (a polyester film having a thickness of 12 μm) was laminated to provide a patch preparation.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com