Preparation method of betamethasone dipropionate
A technology of betamethasone dipropionate and propionic anhydride, applied in the directions of steroids, organic chemistry, etc., can solve the problems of high price of 2-methyltetrahydrofuran, unsuitable for commercial production, unable to meet environmental protection requirements, etc. The effect of strong impurity capacity, moderate reaction temperature and convenient feeding
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
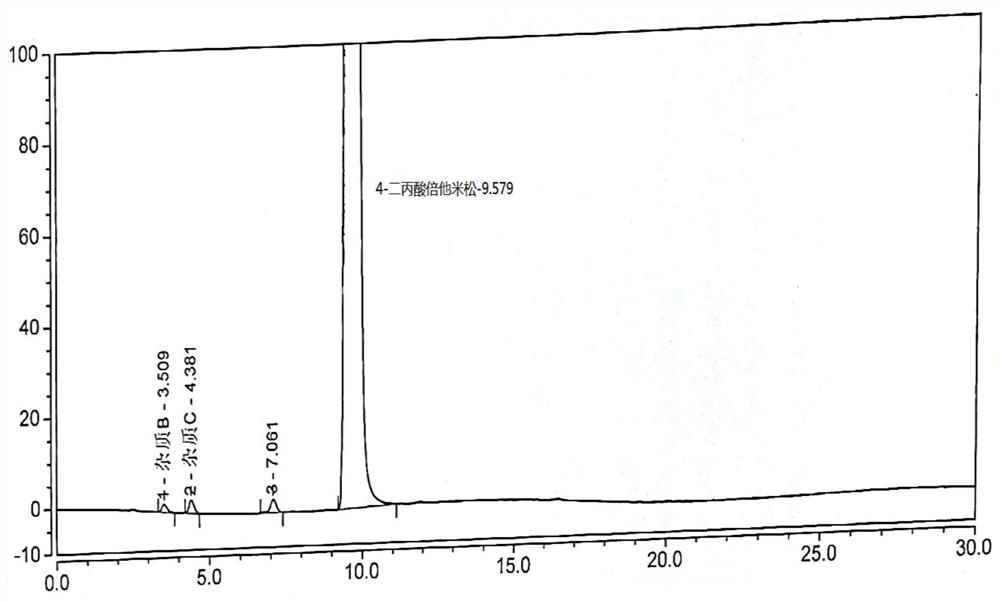
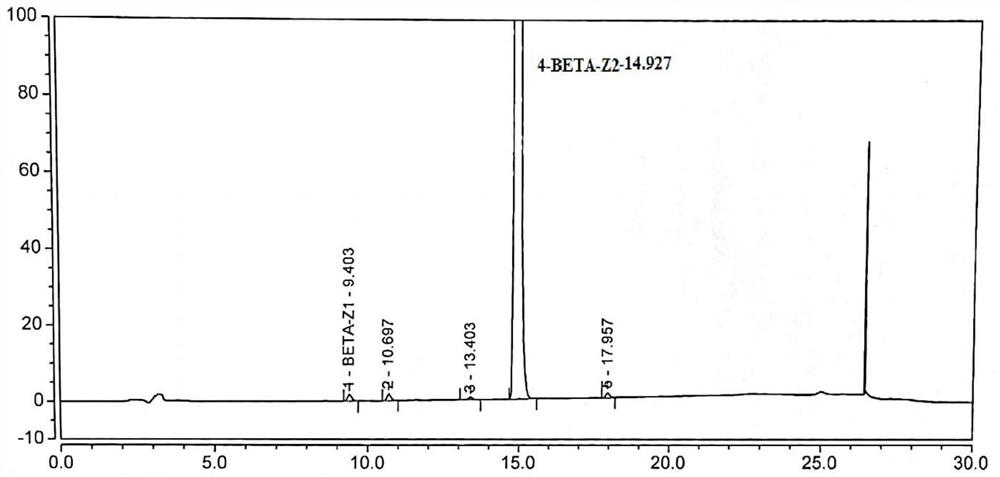
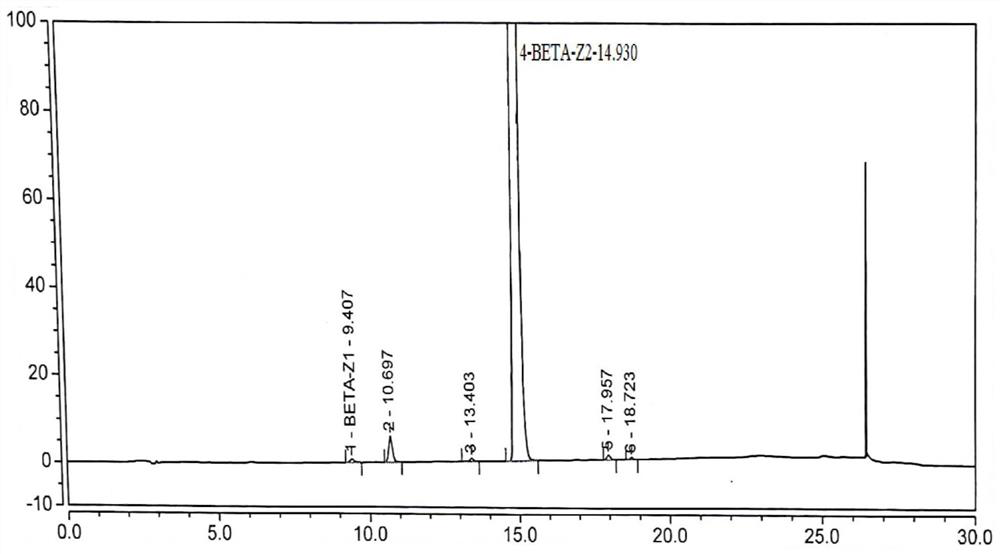
Image
Examples
Embodiment 1 2
[0044] The preparation of embodiment 1 betamethasone dipropionate
[0045] Under nitrogen protection, dichloromethane (14.4kg) was added into the reaction kettle, stirring was started, and betamethasone-17-propionate (compound of formula II, 0.90kg, 2.0mol, 1.0eq), DMAP (catalyst) ( 61.3 g, 0.5 mol, 0.25 eq). Cool down to 0-10°C, add propionic anhydride (313g, 2.4mol, 1.2eq) and dichloromethane (1.197kg) solution, react for 3-5 hours, add water (4.5kg) to quench, let stand to separate , the aqueous layer was discarded, the organic layer was washed 3 times with water, dried with anhydrous sodium sulfate (0.27kg), filtered, and the filtrate was collected, concentrated under reduced pressure (temperature 40°C ~ 50°C, vacuum degree -0.05Mpa ~ -0.10Mpa) After concentrating to a small amount of liquid remaining, add n-hexane (0.594kg), and continue to concentrate to stop flow; add dichloromethane (2.68kg), absolute ethanol (50.4ml), heat to dissolve, add dropwise n-hexane (2.66kg),...
Embodiment 2 2
[0047] The preparation of embodiment 2 betamethasone dipropionate
[0048] Under the protection of nitrogen, add dichloromethane (493ml) into the reaction kettle, start stirring, add betamethasone-17-propionate (compound of formula II, 44.85g, 0.1mol, 1.0eq), DMAP (1.2g, 0.01 mol, 0.1 eq). Cool down to 15-25°C, add a solution of propionic anhydride (15.6g, 0.12mol, 1.2eq) and dichloromethane (45ml), react for 3-5 hours, add water (250ml) to quench, stand to separate layers, The aqueous layer was discarded, the organic layer was washed 3 times with water, dried by adding anhydrous sodium sulfate (25g), filtered, and the filtrate was collected, concentrated under reduced pressure (temperature 40℃~50℃, vacuum degree -0.05Mpa~-0.10Mpa), concentrated After a small amount of liquid remained, add n-hexane (50ml) and continue to concentrate to stop flow, then add n-hexane (50ml) and concentrate to stop flow; add dichloromethane (101ml) and absolute ethanol (2.5ml), heat to dissolve, ...
Embodiment 3 2
[0050] The preparation of embodiment 3 betamethasone dipropionate
[0051] Under the protection of nitrogen, add dichloromethane (493ml) into the reaction kettle, start stirring, add betamethasone-17-propionate (compound of formula II, 44.85g, 0.1mol, 1.0eq), DMAP (6.1g, 0.05 mol, 0.5eq). Cool down to -10-0°C, add propionic anhydride (15.6g, 0.12mol, 1.2eq) and dichloromethane (45ml) solution, react for 3-5 hours, add water (250ml) to quench, let stand and separate , the aqueous layer was discarded, the organic layer was washed 3 times with water, dried with anhydrous sodium sulfate (25g), filtered, and the filtrate was collected, concentrated under reduced pressure (temperature 40°C ~ 50°C, vacuum -0.05Mpa ~ -0.10Mpa), After concentrating to a small amount of liquid, add n-hexane (50ml) and continue to concentrate until the flow is cut off, then add n-hexane (50ml) and concentrate to stop the flow; add dichloromethane (101ml) and absolute ethanol (2.5ml) and heat to dissolve...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com