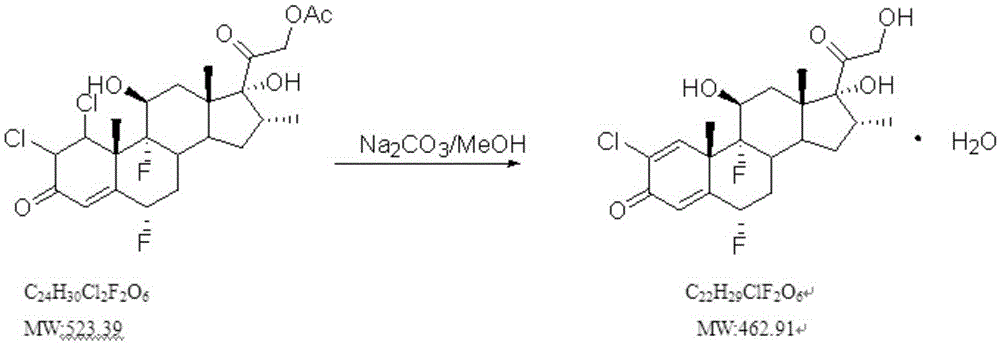
One-step method for synthesizing halometasone from diclomethasone ethyl ester
A technology of diclomethasone ethyl ester and halometasone, applied in the direction of steroids, organic chemistry, etc., can solve the problems of unfavorable industrial production, endangering people's health, cumbersome processing process, etc., and achieve low cost and high yield High, simple method and effective effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1: the synthesis of halometasone
[0032] Synthesize halometasone according to the patent document US3652554, take 10g of diclomethasone ethyl ester, dechlorinate with pyridine, and hydrolyze with aqueous sodium bicarbonate solution, 6.9g, yield: 78.01%, purity: 90.32%
Embodiment 2
[0033] Embodiment 2: the synthesis of halometasone
[0034] Take 10.0g of diclomethasone ethyl ester and add it to a 500ml reaction bottle, then add 100.0g of methanol, blow nitrogen into it and stir to dissolve it, drive out all the air in about 10 minutes, and slowly add 10 10.6g of % sodium hydroxide aqueous solution, about 15 minutes to complete the dropwise addition, temperature control 0-10 ℃ to continue the reaction, TLC detection, about 4.5h to complete the reaction, after the reaction is completed, use glacial acetic acid to control the pH at 6-7, drop quickly Purified water 50.0g, continue temperature control 0-10°C to crystallize for 1h, filter, wash with purified water 20g / time for three times, and vacuum dry at 45°C for 20h to obtain 8.1g light yellow solid powder, yield 91.69%, product structure identification data as follows:
[0035] ESI: molecular ion peak [M+Na] + for 467.1
[0036] H-NMR (δppm): 7.565, 7.567 (d, 1H), 6.224 (s, 1H), 5.610-5.638, 5.691-5.71...
Embodiment 3
[0038] Embodiment 3: the synthesis of halometasone
[0039] Take 10.0g of diclomethasone ethyl ester and add it to a 500ml reaction bottle, then add 100.0g of methanol, blow nitrogen into it and stir to dissolve it, drive out all the air in about 10 minutes, and slowly add 10 % Potassium hydroxide aqueous solution 14.8g, about 15 minutes to complete the dropwise addition, temperature control 0-10 ℃ to continue the reaction, TLC detection, about 4.5h to complete the reaction, after the reaction is completed, use glacial acetic acid to control the pH at 6-7, quickly add dropwise Purified water 50.0g, continue to crystallize at 0-10°C for 1h, filter, wash with purified water 20g / time for three times, and vacuum dry at 45°C for 20h to obtain 7.2g light yellow solid powder, yield 92.72%
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com