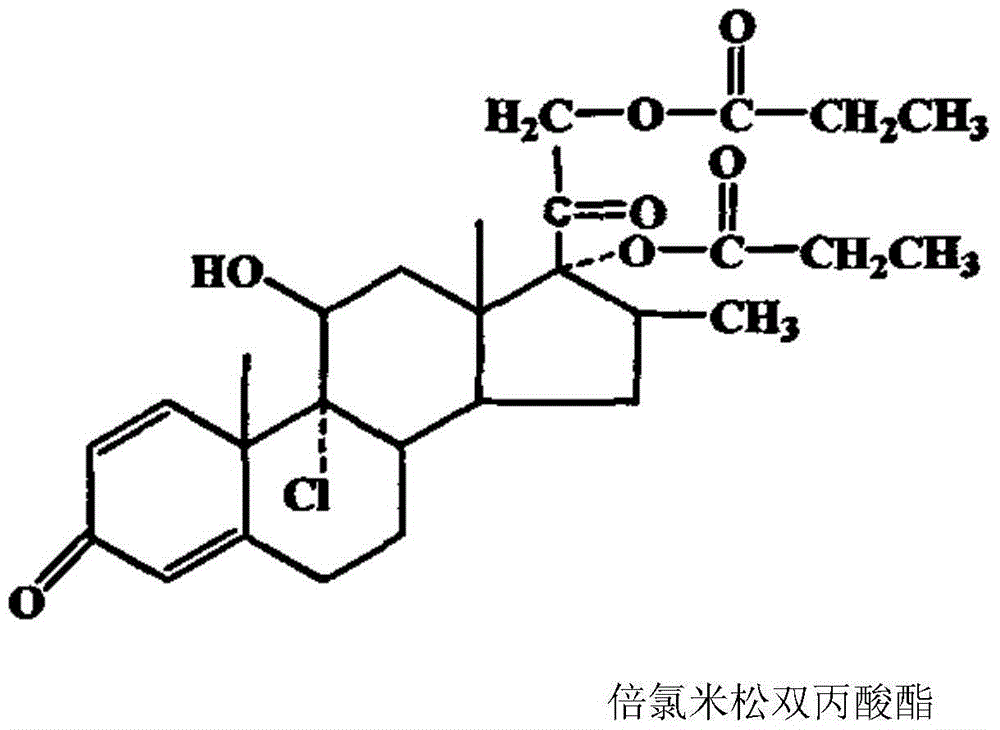
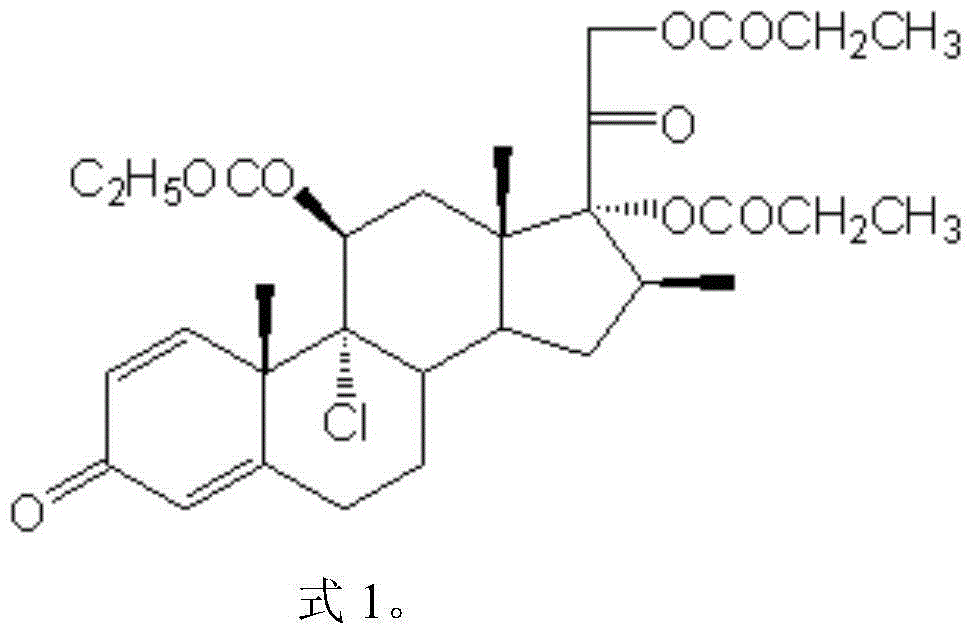
Synthetic method of beclomethasone dipropionate
A technology of dipropionate and beclomethasone, applied in the field of synthesis of beclomethasone dipropionate in aqueous solution of gluconic acid
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Mix 1mmol of beclomethasone 11,17,21-tripropionate in 50ml of organic solvent and 50% aqueous solution of gluconic acid, and react at the specified temperature until the end of the reaction (1 hour interval between beclomethasone 17,21-dipropionic acid The ester content rises and changes less than 1%), concentrate under reduced pressure until there is no organic solvent smell, cool down to 0°C, and dilute in ice water. Filter and dry to obtain beclomethasone 17,21-dipropionate.
[0045]
Embodiment 2
[0047]Mix 1mmol of beclomethasone 11,17,21-tripropionate in 50ml of organic solvent and 50% aqueous solution of gluconic acid, and react at the specified temperature until the end of the reaction (1 hour interval between beclomethasone 17,21-dipropionic acid The ester content rises and changes less than 1%), concentrate under reduced pressure until there is no organic solvent smell, cool down to 0°C, and dilute in ice water. Filter and dry to obtain beclomethasone 17,21-dipropionate.
[0048]
Embodiment 3
[0050] Mix 1mmol of beclomethasone 11,17,21-tripropionate in 50ml of organic solvent and 50% aqueous solution of glucono-δ-lactone, and react at the specified temperature until the end of the reaction (interval of 1 hour for beclomethasone 17, 21-dipropionate content increase and change less than 1%), concentrated under reduced pressure until there is no organic solvent smell, cooled to 0 ℃, diluted in ice water. Filter and dry to obtain beclomethasone 17,21-dipropionate.
[0051]
[0052]
[0053] The average reaction time in embodiment 3 is extended 2 hours compared with embodiment 2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com