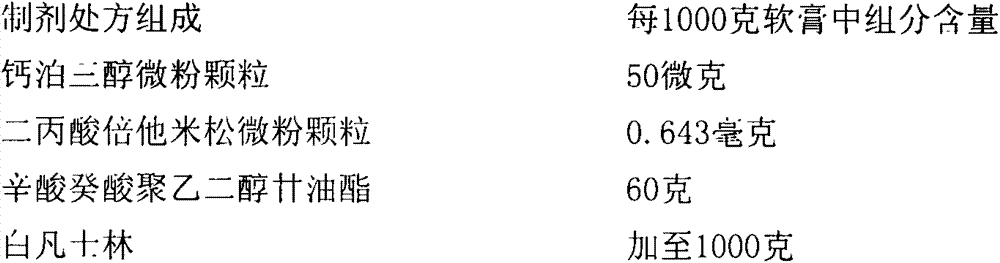Calcipotriol betamethasone ointment and preparation method thereof
A technology of calcipotriol and betamethasone, applied in the field of medicine, can solve the problems of increased impurities, phase separation, uneven content and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Recipe for Ointment 1
[0052]
[0053] making process:
[0054] (a) Micronizing calcipotriol and betamethasone until 90% of the particle size is less than 15 microns, and the particle size range is between 5-110 microns.
[0055] (b) Add (a) to caprylic capric acid macrogol glyceride under high-speed cutting and homogenization, and the temperature is controlled at 40-60°C
[0056] (c) Melt white vaseline at 80°C and keep warm at 70°C
[0057] (d) While stirring continuously, mix the material obtained in step (b) and (c) homogeneously, and control the temperature at 55-70°C
[0058] (e) Cool down to 25-35° C., and pack to obtain calcipotriol-betamethasone ointment.
[0059] The ointment was placed at 40°C and 50°C for 10 days respectively, and the properties of the ointment and the related substances and contents of betamethasone dipropionate were investigated. See Table 1 and Table 2 for the results.
Embodiment 2
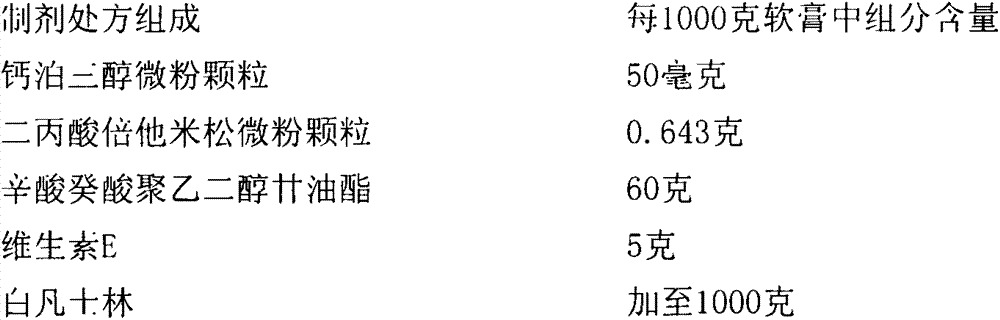
[0061] Recipe for ointment 2
[0062]
[0063] making process:
[0064] (a) Micronizing calcipotriol and betamethasone until 90% of the particle size is less than 15 microns, and the particle size range is between 5-50 microns.
[0065] (b) Add (a) to caprylic capric acid macrogol glyceride under high-speed cutting and homogenization, and the temperature is controlled at 40-60°C
[0066] (c) Melt white vaseline at 80°C and keep warm at 70°C
[0067] (d) Add vitamin E to (c) and stir well.
[0068] (e) While stirring continuously, mix the material obtained in step (b) and (d) homogeneously, and control the temperature at 55-70°C
[0069] (f) cooling down to 25-35° C., and dispensing to obtain calcipotriol-betamethasone ointment.
[0070] The ointment was placed at 40°C and 50°C for 10 days respectively, and the properties of the ointment and the related substances and contents of betamethasone dipropionate were investigated. See Table 1 and Table 2 for the results.
Embodiment 3
[0072] Recipe for ointment 3
[0073]
[0074] making process:
[0075] (a) Micronizing calcipotriol and betamethasone until 90% of the particle size is less than 15 microns, and the particle size range is 5-15 microns.
[0076] (b) Add (a) to caprylic capric acid macrogol glyceride under high-speed cutting and homogenization, and the temperature is controlled at 40-60°C
[0077] (c) Melt white vaseline at 80°C and keep warm at 70°C
[0078] (d) Add vitamin E and ethylparaben to (c) and stir well.
[0079] (e) While stirring continuously, mix the material obtained in step (b) and (d) homogeneously, and control the temperature at 55-70°C
[0080] (f) cooling down to 25-35° C., and dispensing to obtain calcipotriol-betamethasone ointment.
[0081] The ointment was placed at 40°C and 50°C for 10 days respectively, and the properties of the ointment and the related substances and contents of betamethasone dipropionate were investigated. See Table 1 and Table 2 for the res...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com