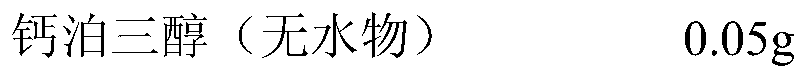A pharmaceutical composition for external use
A technology of externally used drugs and compositions, applied in the field of medicine, which can solve problems such as unstable ointment formulations, uneven content, and increased impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
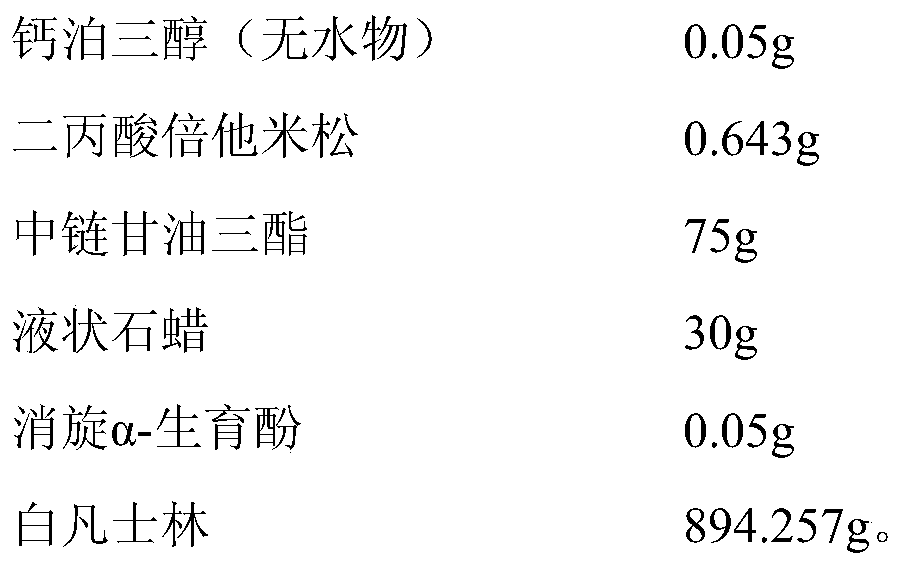
[0036] prescription:
[0037]
[0038] Preparation Process:
[0039] (1) Weigh the prescription amount of white petrolatum, heat it in a 60℃ water bath until it melts completely, and cool it down to 45-50℃ for use;
[0040] (2) Weigh the prescription amount of liquid paraffin, add the prescription amount of racemic α-tocopherol, and stir until the dissolution is complete; add the prescription amount of betamethasone dipropionate (90% particle size <15μm), stir until the dispersion is uniform, and set aside;
[0041] (3) Weigh the prescription amount of medium-chain triglycerides, add the prescription amount of calcipotriol, heat it in a water bath at 50°C until it is completely dissolved, and set aside;
[0042] (4) Add the products of steps (2) and (3) to the product of step (1) and emulsify for 15 minutes at a speed of 4000 rpm;
[0043] (5) After emulsification is finished, the temperature is lowered to 25-35°C, and the product is divided into aliquots and ready to be obtained.
Embodiment 2
[0045] prescription:
[0046]
[0047] Preparation Process:
[0048] (1) Weigh the prescription amount of white petrolatum, heat it in a 60℃ water bath until it melts completely, and cool it down to 45-50℃ for use;
[0049] (2) Weigh the prescription amount of liquid paraffin, add the prescription amount of racemic α-tocopherol, and stir until the dissolution is complete; add the prescription amount of betamethasone dipropionate (90% particle size <15μm), stir until the dispersion is uniform, and set aside;
[0050] (3) Weigh the prescription amount of medium-chain triglycerides, add the prescription amount of calcipotriol, heat it in a water bath at 50°C until it is completely dissolved, and set aside;
[0051] (4) Add the products of steps (2) and (3) to the product of step (1) and emulsify for 15 minutes at a speed of 4000 rpm;
[0052] (5) After emulsification is finished, the temperature is lowered to 25-35°C, and the product is divided into aliquots and ready to be obtained.
Embodiment 3
[0054] prescription:
[0055]
[0056] Preparation Process:
[0057] (1) Weigh the prescription amount of white petrolatum, heat it in a 60℃ water bath until it melts completely, and cool it down to 45-50℃ for use;
[0058] (2) Weigh the prescription amount of liquid paraffin, add the prescription amount of racemic α-tocopherol, and stir until the dissolution is complete; add the prescription amount of betamethasone dipropionate (90% particle size <15μm), stir until the dispersion is uniform, and set aside;
[0059] (3) Weigh the prescription amount of medium-chain triglycerides, add the prescription amount of calcipotriol, heat it in a water bath at 50°C until it is completely dissolved, and set aside;
[0060] (4) Add the products of steps (2) and (3) to the product of step (1) and emulsify for 15 minutes at a speed of 4000 rpm;
[0061] (5) After emulsification is finished, the temperature is lowered to 25-35°C, and the product is divided into aliquots and ready to be obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com