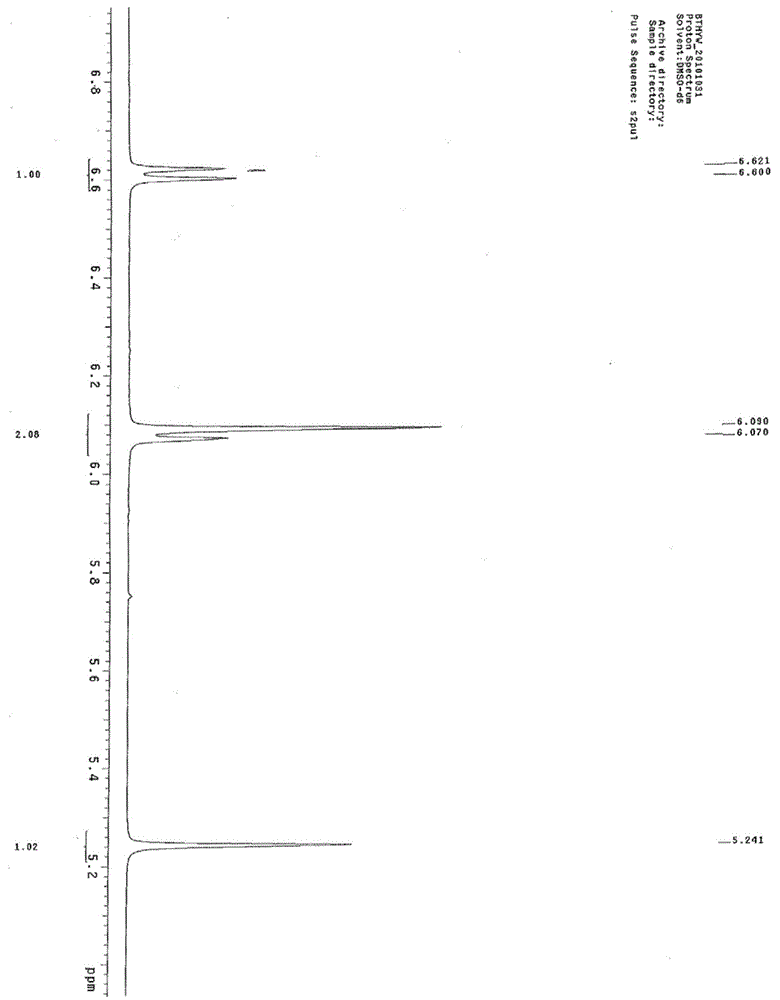
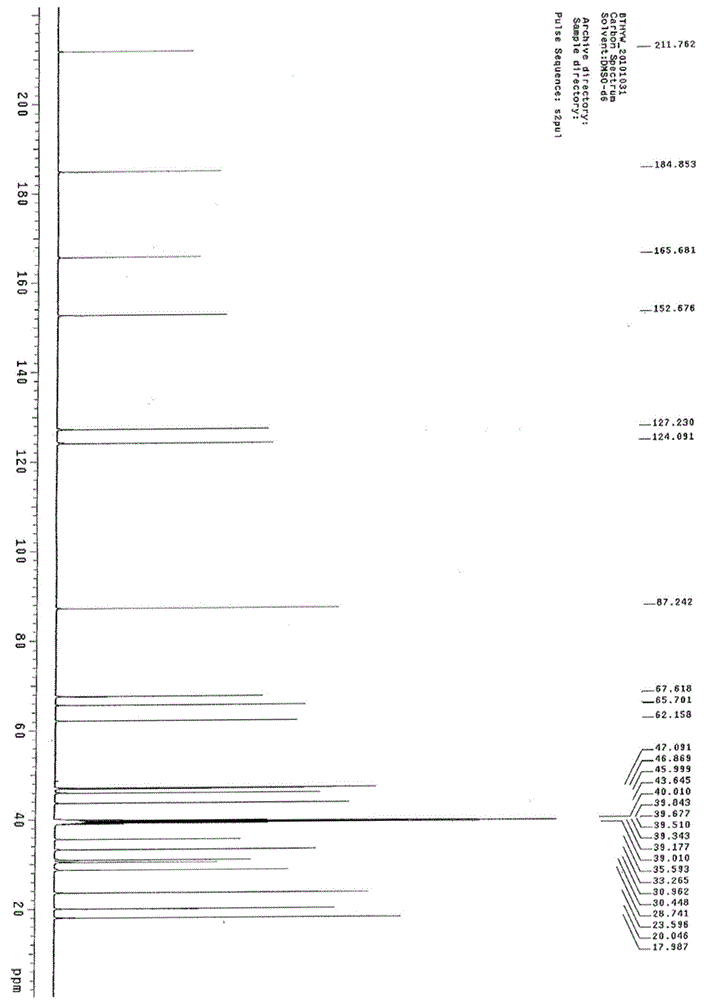
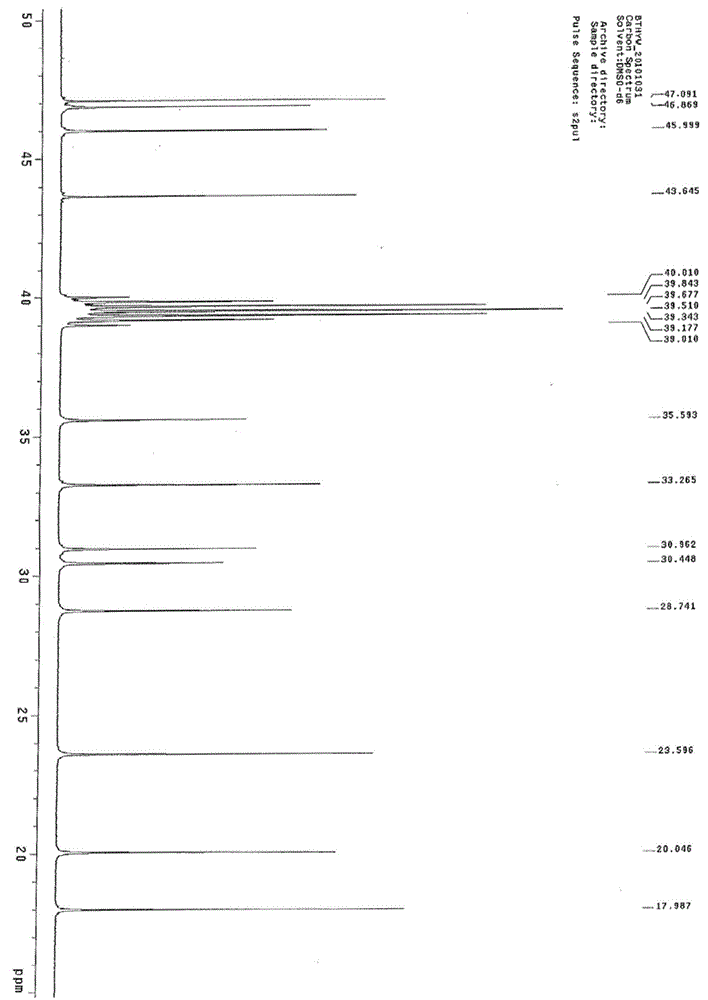
Synthesis method of betamethasone epoxy hydrolyzate
A technology of betamethasone and synthetic methods, applied in the direction of steroids, organic chemistry, etc., can solve the problems of product yield loss, hydrolysis run-off, etc., and achieve low production costs, reduced hydrolysis, and reduced raw and auxiliary materials and power costs Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] A kind of synthetic method of betamethasone epoxy hydrolyzate, comprises the steps:
[0051] (1) take betamethasone digest as raw material, prepare betamethasone bromide through bromohydroxylation reaction, and the reaction process is:
[0052] Add 118L of dioxane and 32kg of betamethasone to a dry bromohydrin reaction tank, blow nitrogen gas, and stir until it dissolves; control the temperature between 10 and 18°C, slowly add 4.1L of perchloric acid, and control When the temperature is between 10 and 20°C, add 18.24kg of beta 101 (i.e. N-bromosuccinimide), after the addition, keep it at 10°C for 40 minutes, then add 104L of methanol and stir for 8 minutes; Pull the reaction solution into the existing sodium sulfite solution (dissolve 7-7.36kg of sodium sulfite in 40L of water) and 1344L of water at an internal temperature of 5-10°C for water analysis in a bromine water analysis tank, and control the water analysis temperature at 5-20°C Between (use starch-potassium io...
Embodiment 2
[0057] A kind of synthetic method of betamethasone epoxy hydrolyzate, comprises the steps:
[0058] (1) take betamethasone digest as raw material, prepare betamethasone bromide through bromohydroxylation reaction, and the reaction process is:
[0059] Add 115L of dioxane and 32kg of betamethasone to the dry bromohydrin reaction tank, blow nitrogen gas, and stir until it dissolves; control the temperature between 10 and 18°C, slowly add 4.1L of perchloric acid, and control When the temperature is between 10 and 20°C, add 18kg of Beta 101 (i.e., N-bromosuccinimide), and after the addition is completed, keep the temperature at 20°C for 20 minutes, then add 100L of methanol, and stir for 5 minutes; Pull the reaction solution into the existing sodium sulfite solution (dissolve 7-7.36kg of sodium sulfite in 40L of water) and 1280L of water at an internal temperature of 5-10°C in the bromohydrin water analysis tank for water analysis, and the water analysis temperature is controlled ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com