Preparation method of betamethasone intermediate
A gas and inert gas technology, applied in the field of preparation of betamethasone intermediates, can solve the problems of high energy consumption, unsafe production, incomplete reaction, etc., and achieve the effects of reducing environmental pollution, saving production costs, and reducing reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
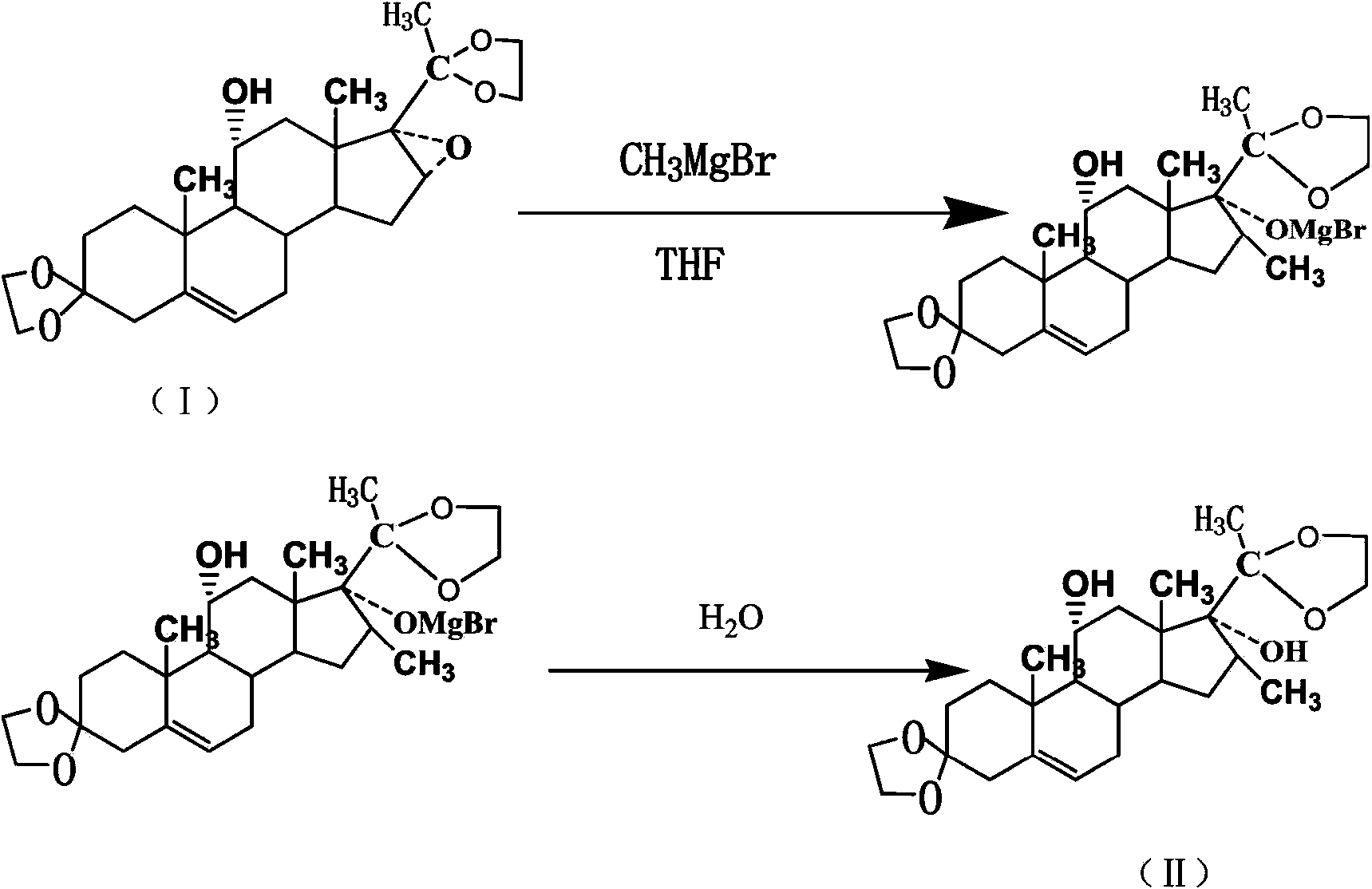
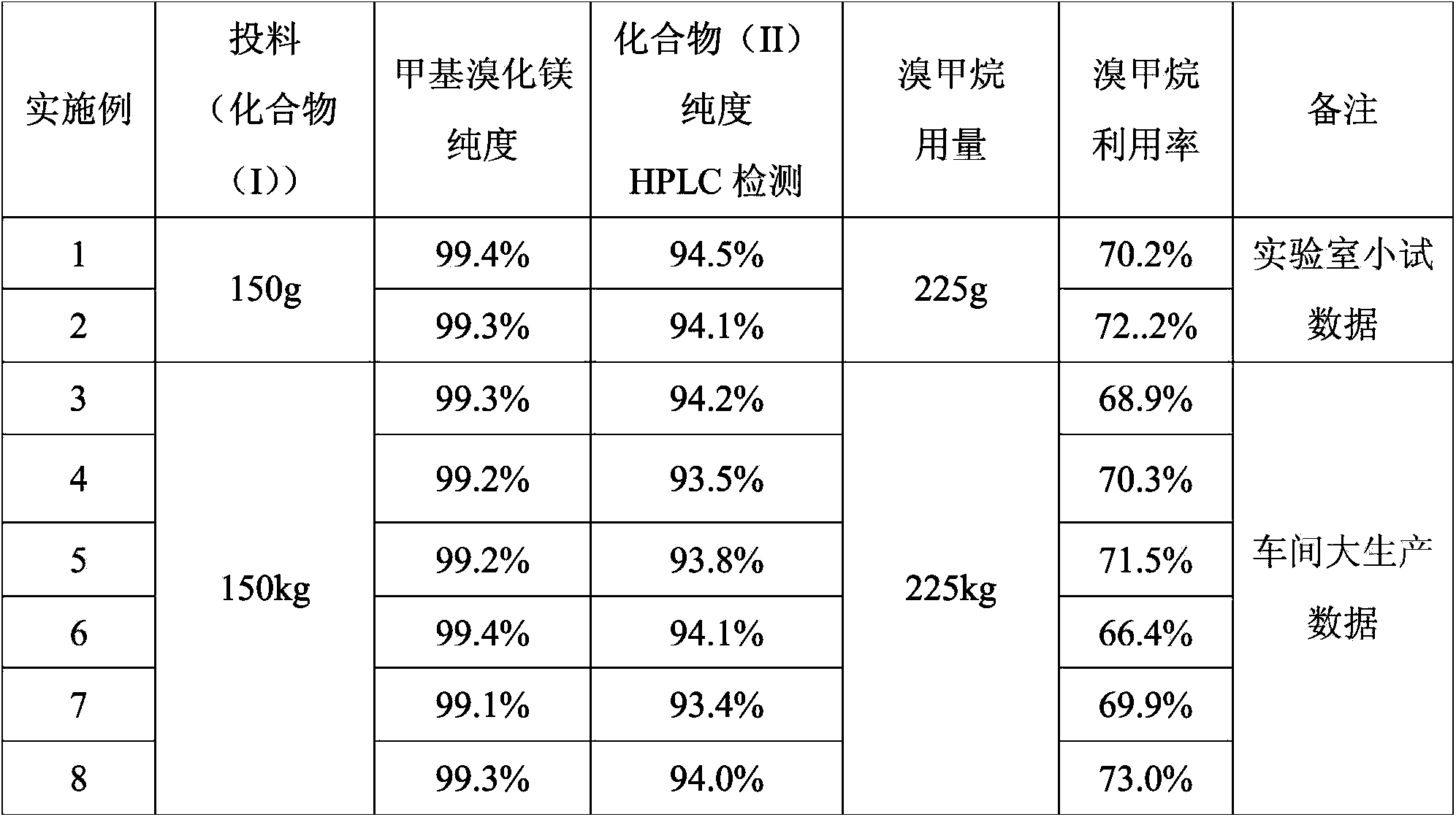
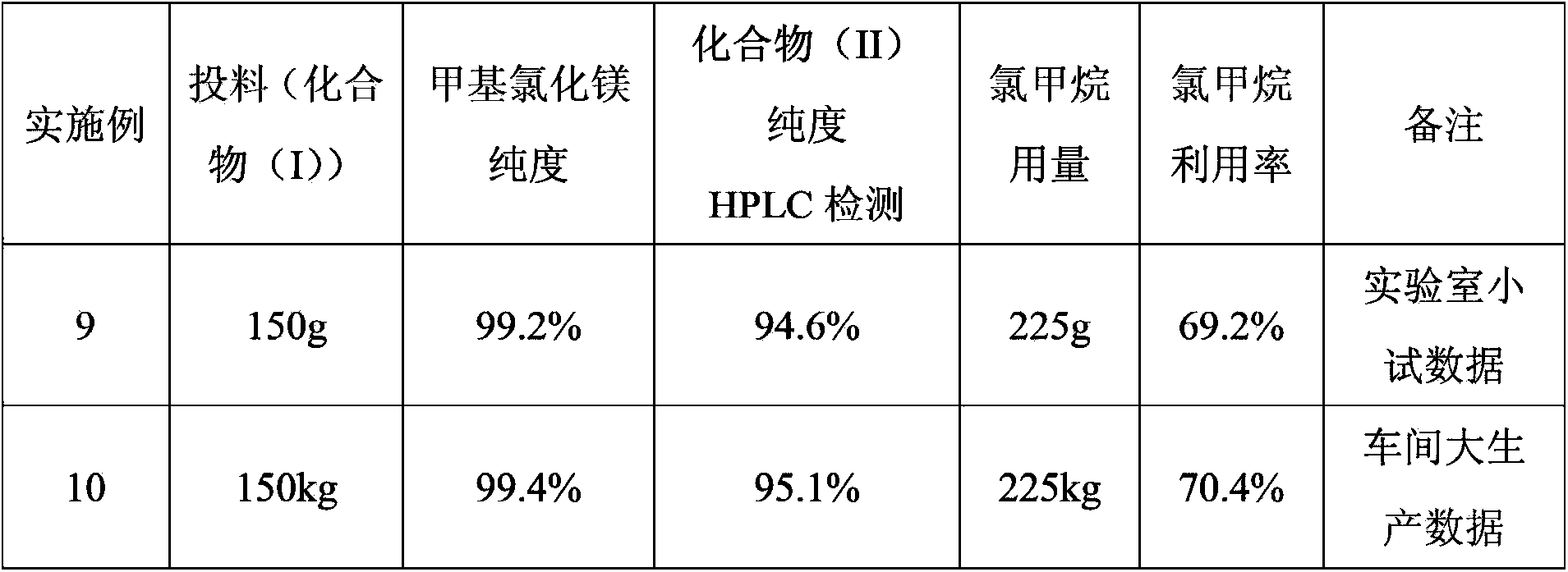
[0028] In a 2000ml four-necked flask, add 1200ml of THF, 58g of magnesium granules, and 0.2g of iodine granules, and replace with nitrogen twice; control the temperature at 40-45°C, stir, and slowly introduce 5g of methyl bromide gas (the gas flow rate is 0.1- 0.5kg / min), after triggering the Grignard reaction, at this temperature, the remaining 220g of methyl bromide gas was passed into the flask for about 12 hours to react. Concentrate under high pressure, collect the fraction after 60°C, when the concentration temperature rises to 79-85°C, open the nitrogen system, stop the concentration, and naturally cool down to room temperature, take a sample to detect that the content of methylmagnesium bromide is 99.4%, and then slowly dissolve 150g of compound 5 -pregnene-16α, 17α-epoxy-11α-hydroxyl-3,20-diethylene glycol ketal (I) was put into the flask, replaced by nitrogen twice, and heated to 60-90°C for reflux reaction for 15 hours, HPLC detects that the chemical substance (I) i...
Embodiment 2
[0031]In a 2000ml four-necked flask, add 800ml tetrahydrofuran, 100g magnesium granules, 0.6g iodine granules, replace with nitrogen twice; control the temperature at 30-40°C, stir, and slowly introduce 8g of methyl bromide gas (gas flow rate is 0.6-1kg / min) , after triggering the Grignard reaction, at this temperature, pass the remaining 217g of methyl bromide gas into the flask for about 12 hours to react. After passing through, reflux for 3 hours until the magnesium particles are completely dissolved, close the nitrogen valve, heat up and concentrate under normal pressure, and collect 60 For the fraction after ℃, when the concentration temperature rises to 79-85 ℃, turn on the nitrogen system, stop the concentration, and naturally drop to room temperature, take a sample to detect that the content of methylmagnesium bromide is 99.3%, and then slowly add 150g of the compound 5-pregnene- 16α, 17α-epoxy-11α-hydroxyl-3,20-diethylene glycol ketal (I) was dropped into the flask, an...
Embodiment 3
[0034] In a 2000L reactor, after adding 1200L, 58kg of magnesium pellets, and 0.2kg of iodine pellets, replace it with nitrogen twice; control the temperature at 45-50°C, stir, and slowly introduce 5kg of methyl bromide gas (the gas flow rate is 0.5-0.7kg / min) , after triggering the Grignard reaction, at this temperature, the remaining 220kg of methyl bromide gas was passed into the flask for about 12 hours to react, and after completion of the passage, the reaction was refluxed for 3 hours until the magnesium particles were completely dissolved, the nitrogen valve was closed, the temperature was raised and concentrated under normal pressure, and 60 For the fraction after ℃, when the concentration temperature rises to 79-85 ℃, turn on the nitrogen system, stop the concentration, and naturally drop to room temperature, take a sample to detect that the content of methylmagnesium bromide is 99.2%, and then slowly add 150kg of the compound 5-pregnene- 16α, 17α-epoxy-11α-hydroxyl-3,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com