Method for preparing tetraene acetate and derivatives thereof
A technology of tetraene acetate and derivatives, applied in the direction of steroids, organic chemistry, etc., can solve the problems of environmental pollution, unsuitable for large-scale production, high production cost, etc., and achieve easy control of reaction conditions, low cost, and simple operation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
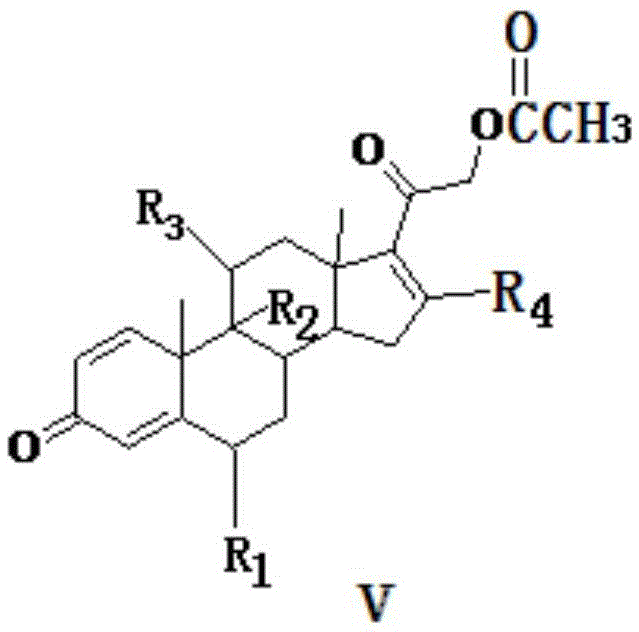
[0035] One embodiment of the preparation method of tetraenyl acetate and its derivative V includes the following steps. The structural formula of tetraenyl acetate and its derivative V is shown in the following formula, wherein R 1 = H, F or CH 3 , R 2 , R 3 =H, OH or double bond, R 4 = H or CH 3 . Among them, R 2 , R 3 =H, OH or double bond represents: R 2 = H or OH, R 3 =H or OH, or R 2 , R 3 = double bond.
[0036]
[0037] Step S100: Under a protective gas atmosphere, compound I is used as a raw material, and compound I is etherified with an etherification reagent to obtain compound II, wherein R 5 , R 6 = H or double bond, R 7 =CH 3 or CH 2 CH 3 , R 1 = H, F or CH 3 , R 2 , R 3 =H, OH or double bond, R 4 = H or CH 3 .
[0038]
[0039] The preparation method uses compound I as a raw material, which is easy to obtain and has low cost.
[0040] Under the action of the etherifying reagent, compound I undergoes an etherification reaction with th...
Embodiment 1
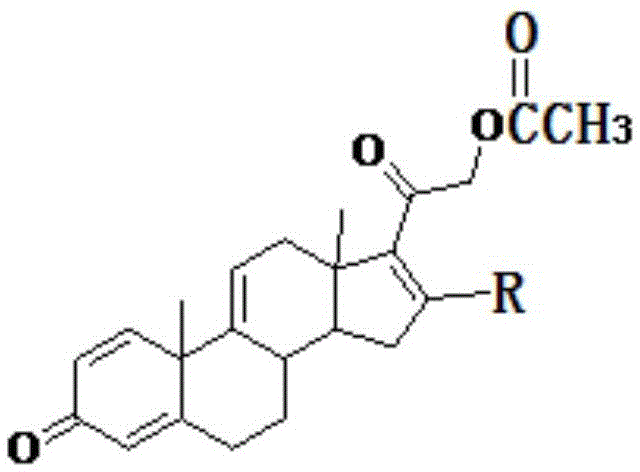
[0104] The structural formulas of raw material compound I and product acetate tetraenyl and its derivative V are shown in the following formula, wherein R 1 = H, R 2 , R 3 = double bond, R 4 = H, R 5 = H, R 6 =H.
[0105]
[0106] Step S100: Under nitrogen protection, add 100 g of compound I, 100 mL of triethyl orthoformate, 200 mL of tetrahydrofuran and 2 g of p-toluenesulfonic acid into a three-neck reaction flask, heat to 40-45° C. and stir for 2 h, and TLC monitors that compound I basically disappears. A mixed solution containing compound II was obtained. Cool the mixture containing compound II to 20-25°C and keep it warm for 30min, add it to 1000mL of ice water to precipitate a solid, keep it warm at 0-10°C and stir for 3h, filter and wash with water to obtain the crudely purified compound II. Dissolve the crudely purified compound II in 300mL of dichloromethane, add 2g of triethylamine to keep it alkaline, add 100mL of methanol, start to concentrate the dichloro...
Embodiment 2
[0111] The specific steps of embodiment 2 are similar to those of embodiment 1, and only the specific steps of step S200 are given below. The difference between embodiment 2 and embodiment 1 is that the structural formulas of the raw material compound I of embodiment 2 and product acetate tetraenyl and derivatives thereof V are shown in the following formula, R 1 = H, R 2 , R 3 = double bond, R 4 =CH 3 , R 5 = H, R 6 =H.
[0112]
[0113] Step S200: under nitrogen protection, add 60mL reagent A (wherein R 8 =CH 2 CH 3 ) and 400mL of THF, cool down to -45°C, slowly add 240mL of butyllithium dropwise, and keep the temperature not higher than -30°C, continue stirring for 30min after the addition of butyllithium is complete, add 100g of compound II, stir, keep warm- Addition reaction occurred at 45° C. for 3 h, and the reaction of compound II was monitored by TLC to obtain a reaction solution containing intermediate IIa. Under the protection of nitrogen, slowly add th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com