Preparation method of key intermediate of dexamethasone and betamethasone
A technology of betamethasone and dexamethasone, which is applied in the field of preparation of intermediates, can solve problems affecting product quality and yield, and achieve the effect of high material conversion rate, high quality and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
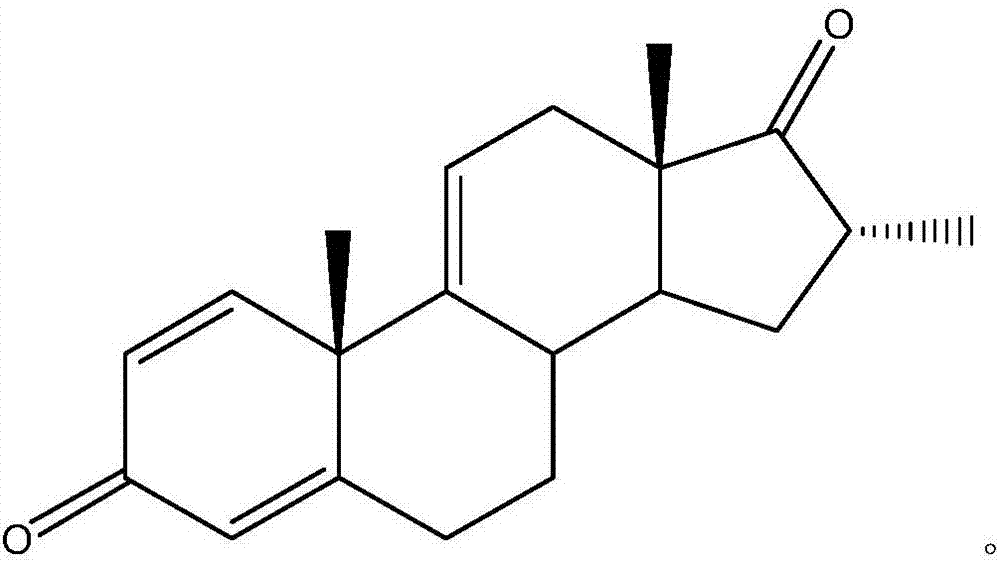
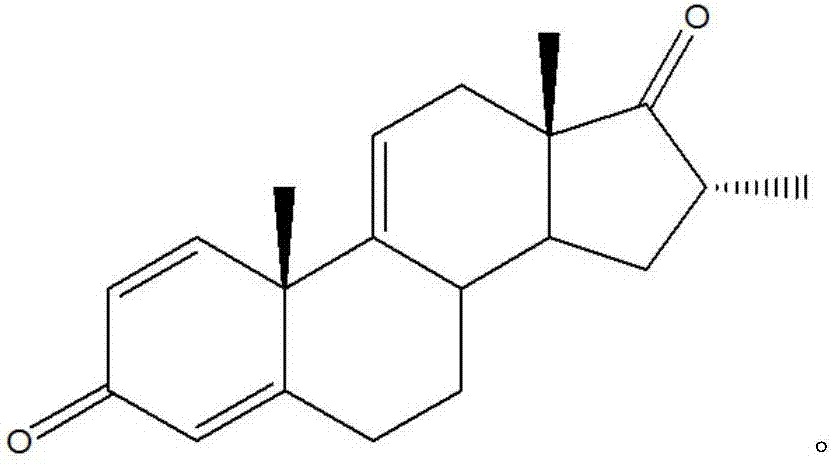
[0026] In the embodiment of the present invention, a kind of preparation method of dexamethasone and betamethasone key intermediate, the structural formula of described dexamethasone and betamethasone key intermediate is as follows:
[0027] The specific steps are as follows:
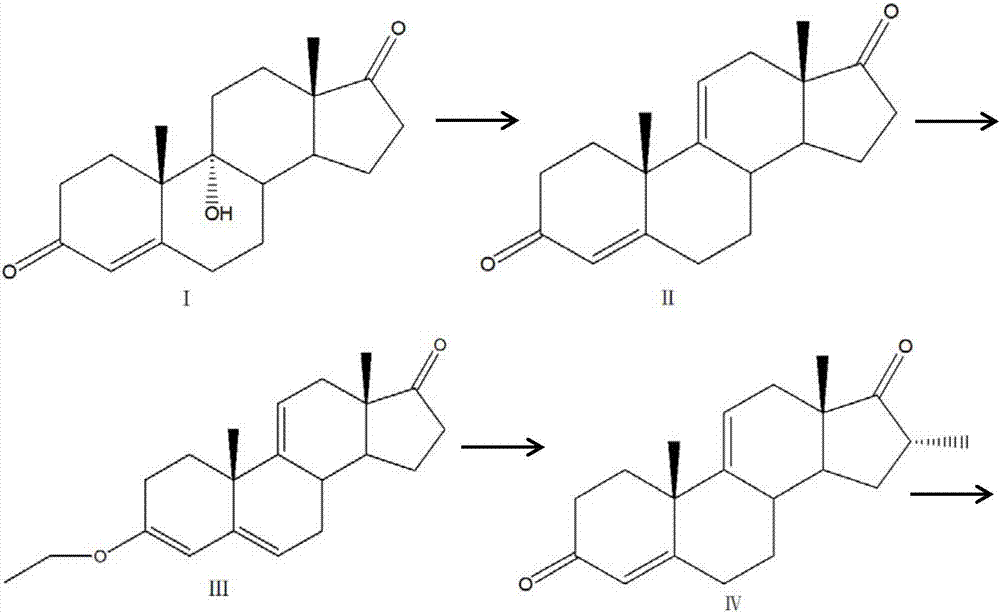
[0028] (1) Compound I first undergoes a dehydration reaction to remove a molecule of water to obtain Compound II;
[0029] (2) Under acidic conditions, compound II is added to ethanol or triethyl orthoformate to obtain 3-ether compound III;
[0030] (3) Under the condition of strong base, methyl bromide is introduced into the solution of 3-ether compound III to obtain 16-methyl compound IV;
[0031] (4) Compound IV prepares key intermediates of dexamethasone and betamethasone by oxidative dehydrogenation in the presence of a metal catalyst; its structural formula is as follows:
[0032]
[0033] In the dehydration reaction, a dehydrating agent is required, and the dehydrating agent is selected fr...
Embodiment 2
[0040] A kind of preparation method of dexamethasone and betamethasone key intermediate, the structural formula of described dexamethasone and betamethasone key intermediate is as follows:
[0041] The specific steps are as follows:
[0042] (1) Compound I first undergoes a dehydration reaction to remove a molecule of water to obtain Compound II;
[0043] (2) Under acidic conditions, compound II is added to ethanol or triethyl orthoformate to obtain 3-ether compound III;
[0044] (3) Under the condition of strong base, methyl bromide is introduced into the solution of 3-ether compound III to obtain 16-methyl compound IV;
[0045] (4) Compound IV prepares key intermediates of dexamethasone and betamethasone by oxidative dehydrogenation in the presence of a metal catalyst; its structural formula is as follows:
[0046]
[0047]
[0048] In the dehydration reaction, a dehydrating agent is required, and the dehydrating agent is selected from phosphorus pentachloride, pho...
Embodiment 3
[0055] A kind of preparation method of dexamethasone and betamethasone key intermediate, the structural formula of described dexamethasone and betamethasone key intermediate is as follows:
[0056]
[0057] The specific steps are as follows:
[0058] (1) Compound I first undergoes a dehydration reaction to remove a molecule of water to obtain Compound II;
[0059] (2) Under acidic conditions, compound II is added to ethanol or triethyl orthoformate to obtain 3-ether compound III;
[0060] (3) Under the condition of strong base, methyl bromide is introduced into the solution of 3-ether compound III to obtain 16-methyl compound IV;
[0061] (4) Compound IV prepares key intermediates of dexamethasone and betamethasone by oxidative dehydrogenation in the presence of a metal catalyst; its structural formula is as follows:
[0062]
[0063] The dehydration reaction needs to use a dehydrating agent, and the dehydrating agent is selected from phosphorus pentachloride, phosphor...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com