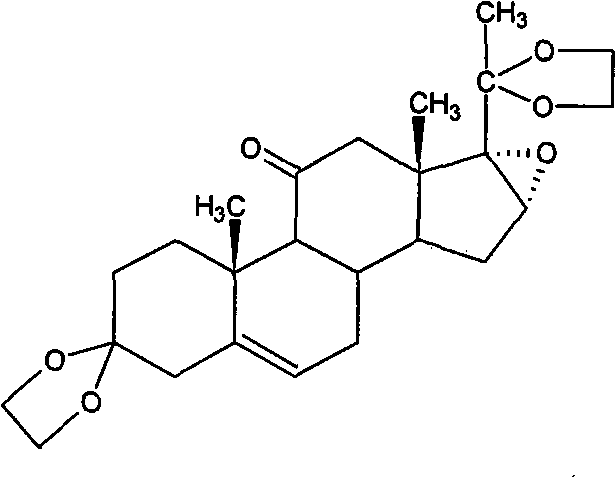
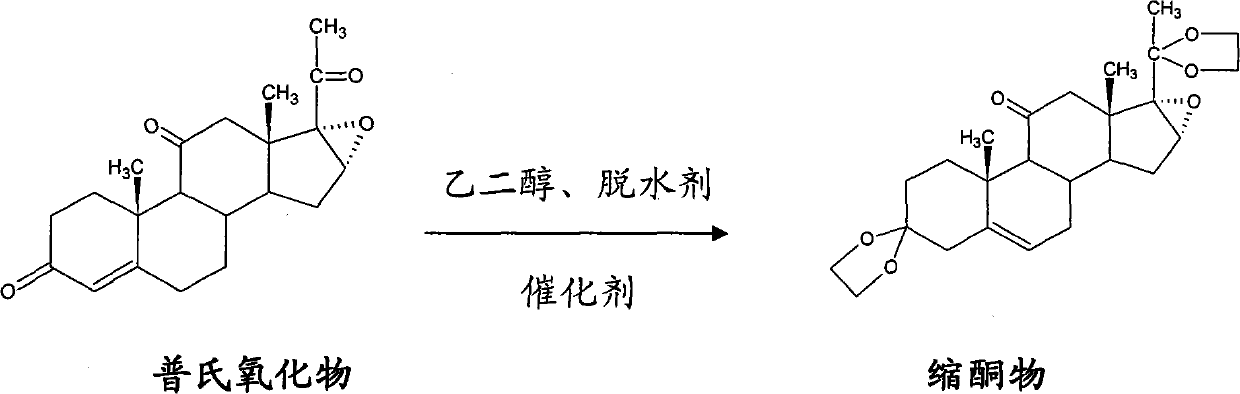
Method for preparing betamethasone ketal by homogeneous reaction at normal temperature
A technology of betamethasone and ketal, which is applied in the field of steroid hormone drug synthesis, can solve the problems of increased quality of side reactions, incomplete reaction, and reduced catalytic effect, so as to reduce the amount of solvents and various materials, and improve the quality of products. The effect of improving quality and yield, and quality standards
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Put 20kg of ethylene glycol, 5kg of triethyl orthoformate, and 0.6kg of boron trifluoride ether into the reaction tank. After stirring evenly, add 10kg of Platinum oxide and 10kg of dichloromethane, and react at room temperature for 2-15 hours. Add triethylamine, the end point is subject to chromatography, and then concentrate, cool down, stand still, shake off the material, and dry to obtain the ketal product.
[0030] After shaking off the material, re-concentrate the filtrate, let it stand, and then throw off the filter to obtain a hemicondensate, which can be re-thrown for ketal reaction, with a yield of 118% and a content of 93.72%.
Embodiment 2
[0032] Put 30kg of ethylene glycol, 30kg of triethyl orthoformate, and 1kg of boron trifluoride ether into the reaction tank. After stirring evenly, add 10kg of Platinum oxide and hemicondensate, and 6kg of dichloromethane, and react at room temperature for 2- After 15 hours, triethylamine was added, the end point was subject to chromatography, and then concentrated, cooled, left standing, shaken, and dried to obtain a ketal product.
[0033] After shaking off the material, re-concentrate the filtrate, let it stand, and then throw off the filter to obtain a hemicondensate, which can be re-thrown for ketal reaction, with a yield of 116% and a content of 91.7%.
Embodiment 3
[0035] Put 60kg of ethylene glycol, 20kg of triethyl orthoformate, and 6kg of boron trifluoride ether into the reaction tank. After stirring evenly, add 10kg of Platinum oxide and hemicondensate, and 15kg of dichloromethane, and react at room temperature for 2- After 15 hours, triethylamine was added, the end point was subject to chromatography, and then concentrated, cooled, left standing, shaken, and dried to obtain a ketal product.
[0036] After shaking off the material, re-concentrate the filtrate, let it stand, and then throw off the filter to obtain a hemicondensate, which can be re-thrown for ketal reaction, with a yield of 115% and a content of 92.3%.
[0037] The properties of the ketal prepared in Examples 1-3 of the present invention: white or off-white crystalline powder. Quality standards for ketals:
[0038] project
[0039] The prepared ketal can be reacted to the final qualified product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com