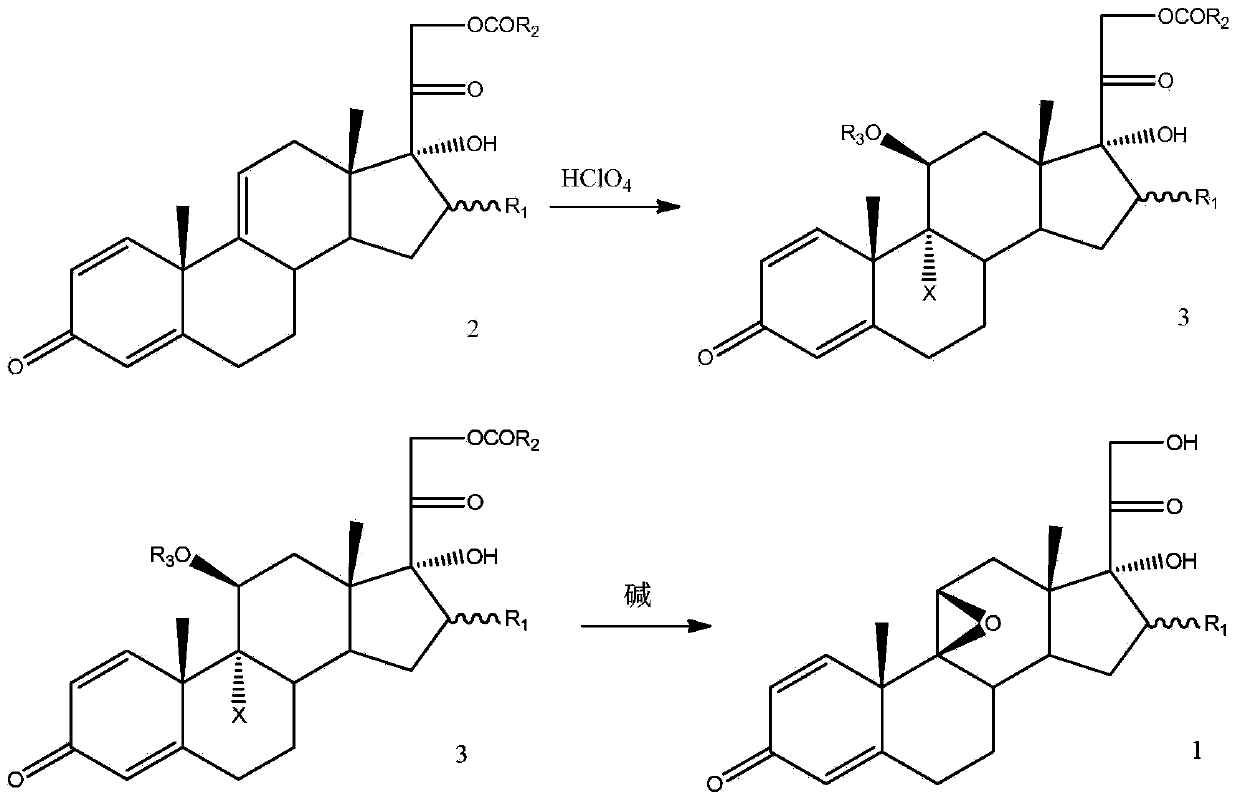
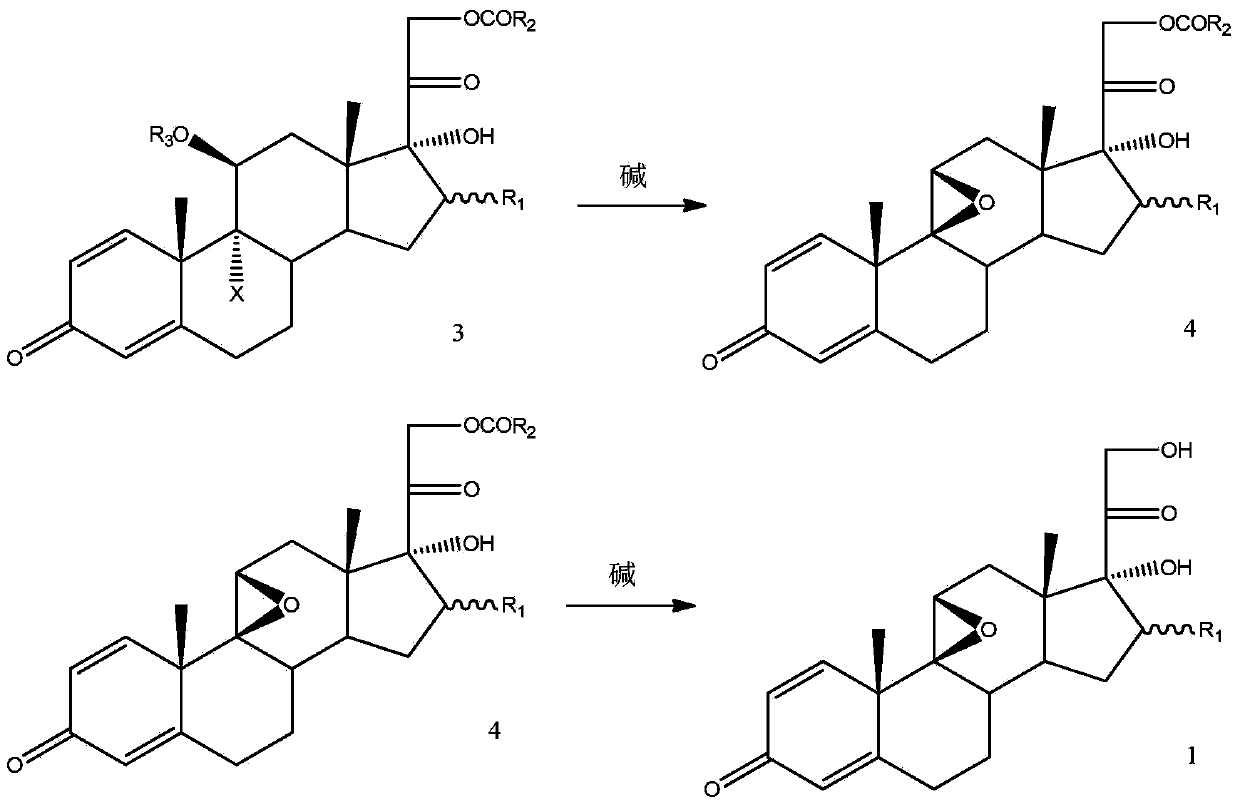
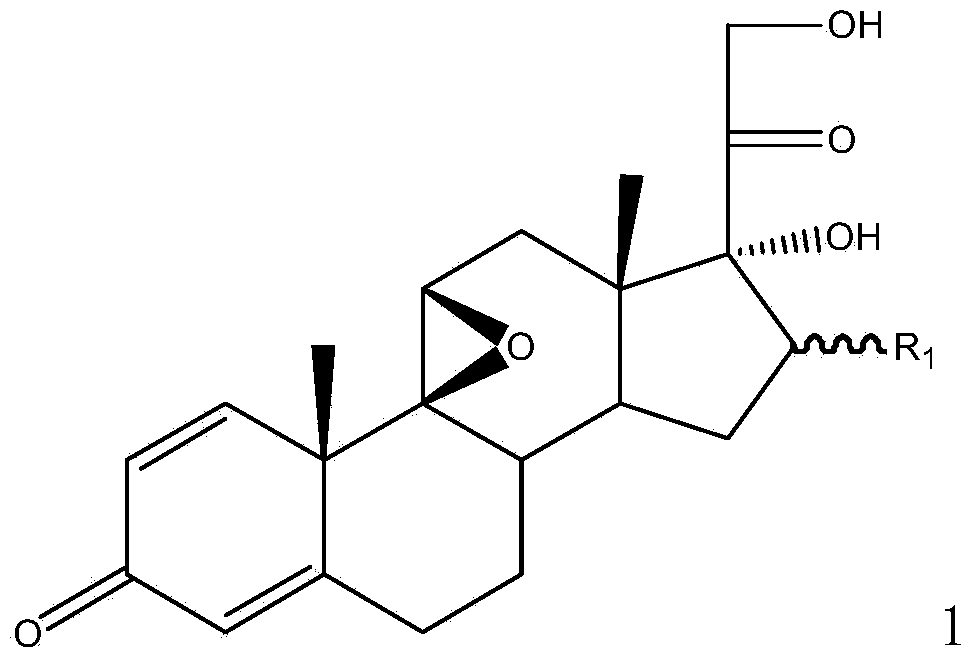
Method for preparing 9,11beta-epoxy steroid compound
A technology of epoxy steroids and compounds, which is applied in the field of preparation of key intermediates 9,11β-epoxy steroids, and can solve problems affecting product quality and yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Example 1: 16β-methyl-9α-bromo-11β-formyloxy-1,4-diene-pregna-11β,17α,21-trihydroxy-3,20-dione-21-b Preparation of esters
[0055]
[0056] Put 10.0g of 16β-methyl-1,4,9-triene-pregna-17α,21-dihydroxy-3,20-diketone-21-acetate and 35.0g of DMF under nitrogen protection , cooled to 0°C, and kept the temperature not exceeding 5°C, add 3.6g of 70% perchloric acid aqueous solution dropwise, and add 5.0g of DBH in 3 times. After reacting for 2 hours, no raw material point was detected by HPLC, 30 g of methanol was added, and stirred for half an hour. Pour the reaction solution into 300g of ice-water mixture, stir at 0-5°C for 1 hour, filter with suction, and dry the filter cake under vacuum at 40°C to obtain 13.0g of 16β-methyl-9α-bromo-11β-formyloxy-1 , 4-diene-pregna-11β,17α,21-trihydroxy-3,20-dione-21-acetate (Formula 3.1), HPLC99.5%.
Embodiment 2
[0057] Example 2: Preparation of 16β-methyl-9,11β-epoxy-1,4-diene-pregna-17α,21-dihydroxy-3,20-dione
[0058]
[0059] Put in 16β-methyl-9α-bromo-11β-formyloxy-1,4-diene-pregna-11β,17α,21-trihydroxy-3,20-dione of formula 3.1 under nitrogen protection 10.0 g of -21-acetate, 100 g of DCM, and 20 g of methanol were cooled to -5°C. 40% sodium hydroxide aqueous solution containing 3.0 g of sodium hydroxide was added dropwise, and the temperature was controlled at -5-0°C to keep the reaction for 2 hours. After no raw material was detected by HPLC, 8 g of glacial acetic acid was added dropwise. Concentrate under reduced pressure at 40°C until the remaining 25g, pour the reaction solution into 200g of ice-water mixture, stir at 0-5°C for 1 hour, and filter with suction to obtain 7.0g of wet crude product. Add the wet crude product, 170g of DCM, and 25g of methanol, stir and heat up to reflux, reflux for half an hour, and heat filter. The filtrate was concentrated under normal pre...
Embodiment 3
[0060] Example 3: Preparation of 16β-methyl-9,11β-epoxy-1,4-diene-pregna-17α,21-dihydroxy-3,20-dione
[0061]
[0062] Put in 16β-methyl-9α-bromo-11β-formyloxy-1,4-diene-pregna-11β,17α,21-trihydroxy-3,20-dione of formula 3.1 under nitrogen protection 10.0 g of -21-acetate and 200 g of methanol were cooled to 0°C. 25% methanol solution containing 2.5g of sodium hydroxide was added dropwise, and the temperature was controlled at 0-5°C to keep the reaction for 2 hours. After HPLC detected that there was no raw material, 8g of glacial acetic acid was added dropwise. Concentrate under reduced pressure at 40°C until about 25g remains, pour the reaction solution into 200g of ice-water mixture, stir at 0-5°C for 1 hour, and filter with suction to obtain 7.0g of wet crude product. Add the wet crude product, 170g of DCM, and 25g of methanol, stir and heat up to reflux, reflux for half an hour, and heat filter. The filtrate was concentrated under normal pressure to an internal tempe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com