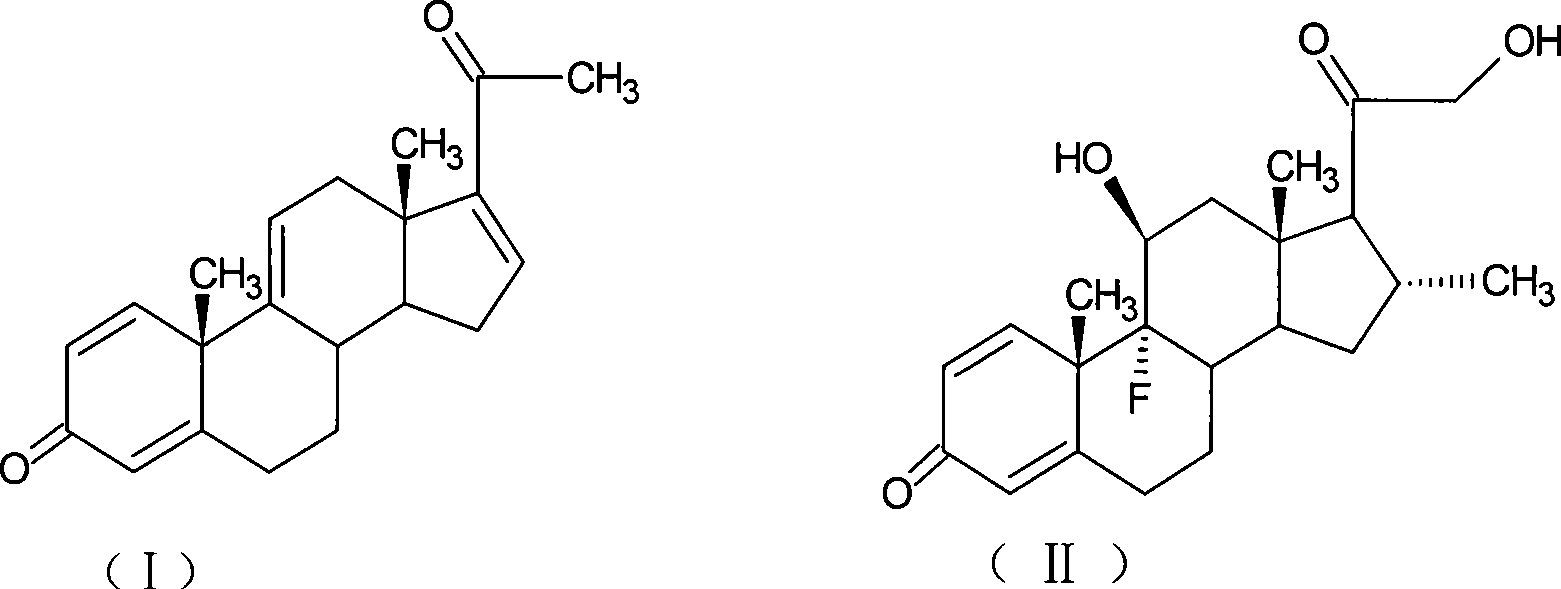
Preparation of desoximetasone
A technology of deoxymethasone and its compound, which is applied in the field of preparation of deoxymethasone, can solve the problems of low yield, long and cumbersome reaction route, etc., and achieve the effect of easy availability of raw materials, reduction of production cost and industrial conditions, and simple circuit
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
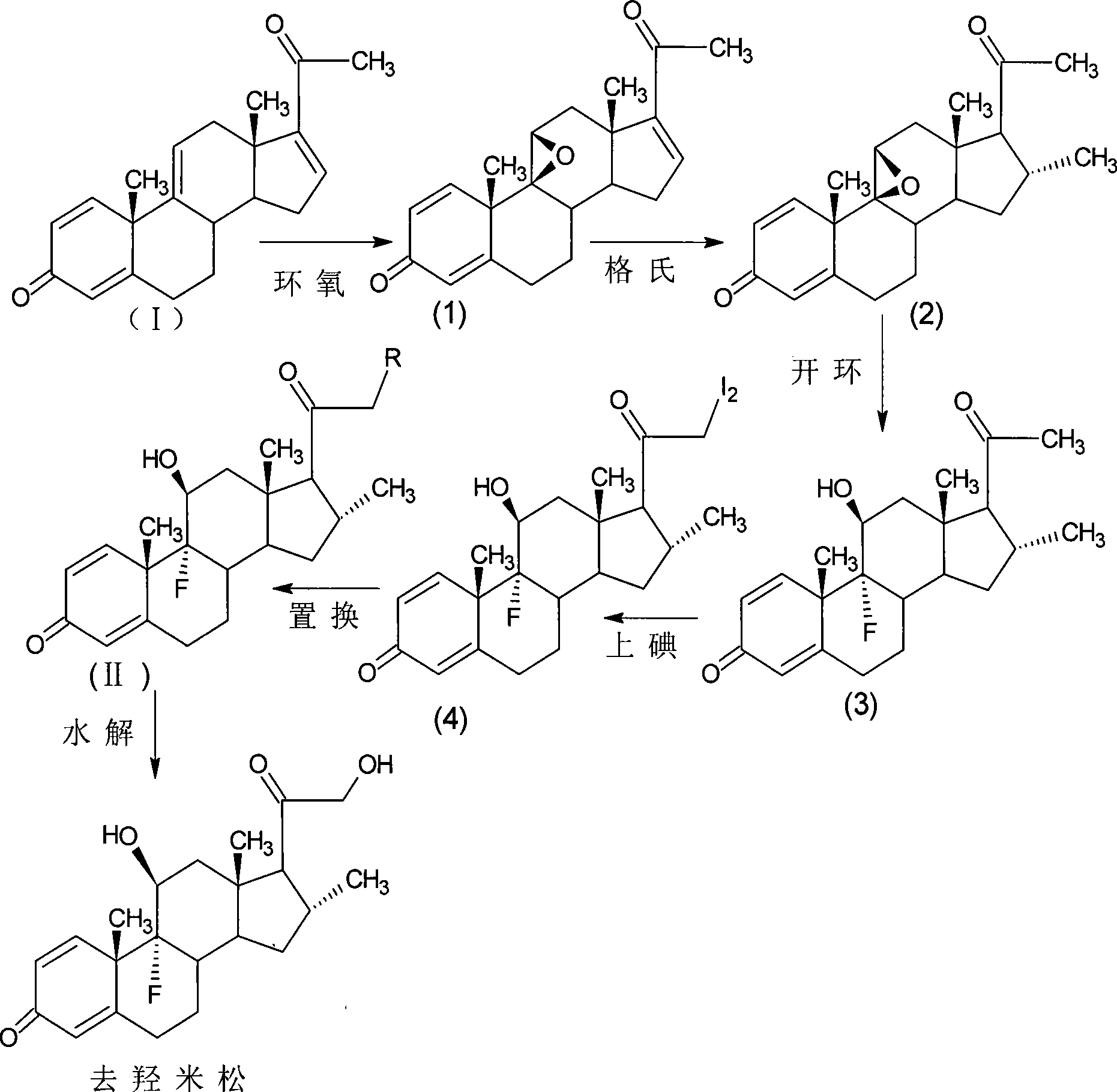
[0069] epoxy reaction
[0070] Add 10g of 1,4,9,16-tetraene-pregna-3,20-dione (CN1896090), 120ml of acetone into the reaction flask, stir, cool down to 0°C, add NBS9g within 30 minutes, keep React at 5-10°C for 2 hours, add 10% sodium carbonate aqueous solution to neutralize to PH=6.5, raise the temperature to 20±2°C, add 10% sodium hydroxide aqueous solution 15ml within 1 hour, and control the temperature at 20-25°C for reaction After 2 hours, acetic acid was neutralized to PH=7, concentrated under reduced pressure until there was no acetone smell, diluted into ice water, filtered, and dried to obtain 10.2 g of 9,11-epoxy-1,4,16-triene-pregnan Stero-3,20-dione (1).
[0071] Grignard reaction
[0072] The configuration of the Grignard reagent: in 20ml of tetrahydrofuran, pass nitrogen, add 0.8g of magnesium strips, pass in methyl bromide gas, heat up and reflux for 0.5h, cool down for later use.
[0073] Add 60ml of tetrahydrofuran, 0.1g of ketone chloride and 10.2g of 9,11...
Embodiment 2
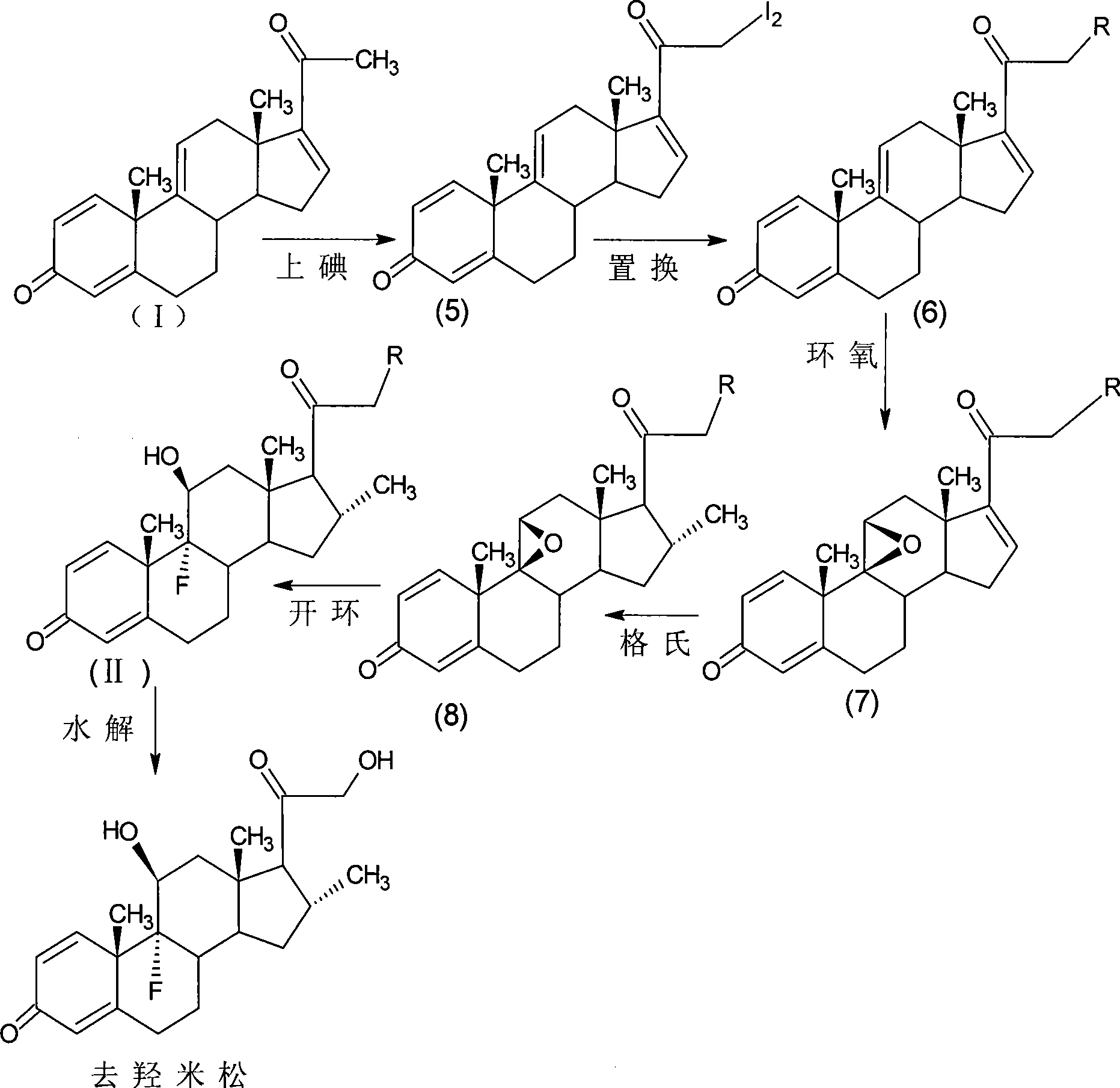
[0084] Iodine reaction:
[0085] Add 150ml of methanol and 6.1g of calcium oxide to the reaction bottle, and 8.5g of anhydrous calcium chloride in 90ml of methanol solution in another volumetric flask, take out 1 / 4 after dissolving, add to the reaction bottle, and dissolve the rest 15.0g of iodine particles, Add 10g of 1,4,9,16-tetraene-pregna-3,20-dione (CN1896090) into the reaction flask, fill with nitrogen, control the temperature at 0±5°C, add iodine solution dropwise, drop for about 3 hours Finished, after another 1 hour of reaction, the reaction solution was diluted in 600ml of 2% ammonium chloride aqueous solution, stirred for 1 hour, left to stand for 1 hour, filtered, washed with water to neutrality, and obtained wet product iodide (5). The product is unstable and does not need to be dried, and the storage time should not be too long, and it is ready for use.
[0086] Replacement reaction:
[0087] Add 40ml of DMF, 1ml of acetic acid, 0.8g of potassium acetate to th...
Embodiment 3
[0099]epoxy reaction
[0100] With the method of Example 1, 10g of 1,4,9,16-tetraene-pregna-3,20-dione (CN1896090) obtained 10.1g of 9,11-epoxy-1,4,16-trione En-pregna-3,20-dione (1).
[0101] Iodine reaction:
[0102] With the method of Example 1, 11.1g of 9,11-epoxy-1,4,16-triene-pregna-3,20-dione (1) was loaded with iodine to obtain wet product iodide 21-biiodo- 9,11-Epoxy-1,4,16-triene-pregna-3,20-dione (9).
[0103] Replacement reaction:
[0104] With the method of Example 1, the iodide was replaced to obtain 11.2g of 21-hydroxyl-9,11-epoxy-1,4,16-triene-pregna-3,20-diketone-21-acetate ( 10).
[0105] Grignard reaction
[0106] With the method of Example 1, 11.2g of 21-hydroxyl-9,11-epoxy-1,4,16-triene-pregna-3,20-diketone-21-acetate (10) Grignard obtained 10.7 g of 16α-methyl-9,11-epoxy-21-hydroxy-1,4-diene-pregna-3,20-dione-21-acetate (11).
[0107] ring opening reaction
[0108] With the method of Example 1, 10.7g of 16α-methyl-9,11-epoxy-21-hydroxyl-1,4-diene...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com