Method for simultaneously detecting two glucocorticoid isomerides in animal-derived food
A technology for glucocorticoids and isomers, applied in the fields of food safety testing and analytical chemistry, can solve the problems of difficult test results, inability to judge mixtures, and high cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
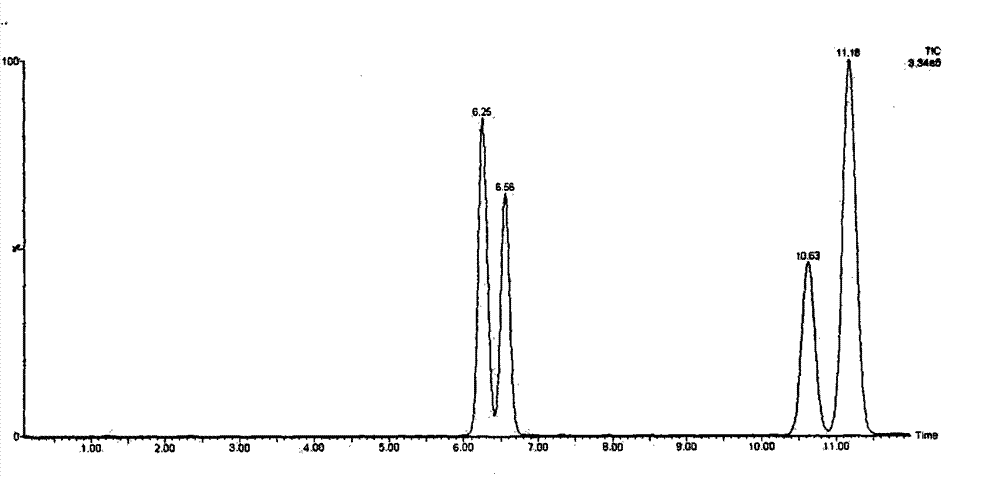
[0041] Determination of betamethasone, dexamethasone, fluorometholone and deaximethasone in embodiment 1 mutton.
[0042] (1) Sample extraction: Weigh 2.5 g of the mixed sample, add 20 mL of methanol, vortex, shake for 15 min, sonicate for 10 min, centrifuge at 10,000 r / min for 5 min, transfer the supernatant to a rotary steamer, and spin evaporate to near dryness below 40°C. Transfer the liquid in the rotary evaporator to a centrifuge test tube, wash the rotary evaporator with 2ml of methanol, transfer the washing solution to the test tube, dilute to 14ml with water, vortex and mix for 2min, centrifuge at 10000r / min for 5min, and collect the supernatant for use.
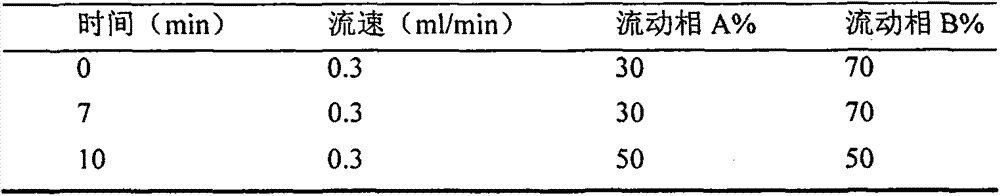
[0043] (2) Purification of the extract: install the solid-phase extraction column, activate it with 5ml of methanol and 5ml of water, put the extract on the column, control the flow rate <2ml / min, rinse with 4ml of eluent and 5ml of n-hexane respectively, and use 6 ml of methanol for elution. The eluate was blown d...
Embodiment 2
[0059] Example 2 Determination of betamethasone, dexamethasone, fluorometholone and deoxymethasone in milk.
[0060] (1) Sample extraction: Weigh 5.0 g of the mixed sample, add 20 mL of methanol, vortex, shake for 15 min, sonicate for 10 min, centrifuge at 10,000 r / min for 5 min, remove the supernatant to a rotary evaporator, and rotatable below 40°C until nearly dry. Transfer the liquid in the rotary evaporator to a centrifuge test tube, wash the rotary evaporator with 2ml of methanol, transfer the washing solution to the test tube, dilute to 14ml with water, vortex and mix for 2min, centrifuge at 10000r / min for 5min, and collect the supernatant for use.
[0061](2) Purification of the extract: install the solid-phase extraction column, activate it with 5ml of methanol and 5ml of water, put the extract on the column, control the flow rate <2ml / min, wash it with 4ml of eluent and 5ml of n-hexane respectively, and use 6ml of methanol eluted. The eluate was blown dry with nitro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com