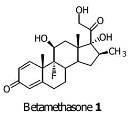
Preparation method of betamethasone intermediate
A technology of betamethasone and intermediates, which is applied in the field of preparation of steroid compounds, can solve the problems of long steps and low yield, and achieve the effects of reducing side reactions, improving yield and quality, and strong operability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] sulfonation elimination reaction
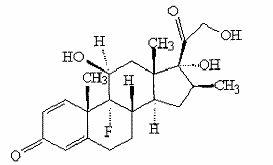
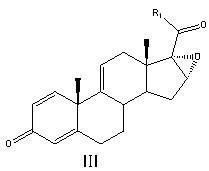
[0043] Put in 50ml acetone and 200ml pyridine, stir, nitrogen protection, then put in 50g compound II (16a,17-epoxy-11a-hydroxyl-1,4-pregnadiene-3,20-dione), cool to -10 Below ℃, add 80ml of methanesulfonyl chloride dropwise, and keep the temperature of the reaction solution not exceeding -10℃ during the whole dropping process. After the dropwise addition, react at a constant temperature of -10°C for 5 hours. After the reaction is complete, slowly add the reaction solution dropwise to a mixed solution consisting of 1000ml of water and 200ml of concentrated hydrochloric acid. After the dropwise addition, stir at a constant temperature of 0°C for 4 hours. hour, filter off-white solid, put it into 300ml of acetic acid, then put into 60g of potassium acetate and 3g of magnesium chloride and heat up to 80°C to react for 2h, after the reaction is complete, slowly drop the reaction solution into 1200ml of water, stir for 1h, filter, and dry ...
Embodiment 2
[0051] sulfonation elimination reaction
[0052] Add 80ml n-butyl ether and 25g imidazole, stir, nitrogen protection, then drop 50g compound II, the structural formula is
[0053]
[0054] Among them, R is β-OH; R 1 for CH 2 COOCH 2 CH 3 ;
[0055] Cool down to below 20°C, add 30g p-toluenesulfonyl chloride, keep the temperature of the reaction solution not exceeding 20°C during the whole adding process. After the addition, react at a constant temperature of 10°C for 7 hours. After the reaction is complete, slowly add the reaction solution dropwise to a mixed solution composed of 1000ml of water and 200ml of concentrated hydrochloric acid. After the addition, stir at a constant temperature of 0°C for 4 hours. Filter the off-white solid, put it into 300ml of formic acid, then put 50g of sodium carbonate and 3.3g of calcium chloride into it and raise the temperature to 60°C for 4 hours. dry to obtain compound III 45g, molar yield 94.3%, by-product △ 1,4,11(12) Accounti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com