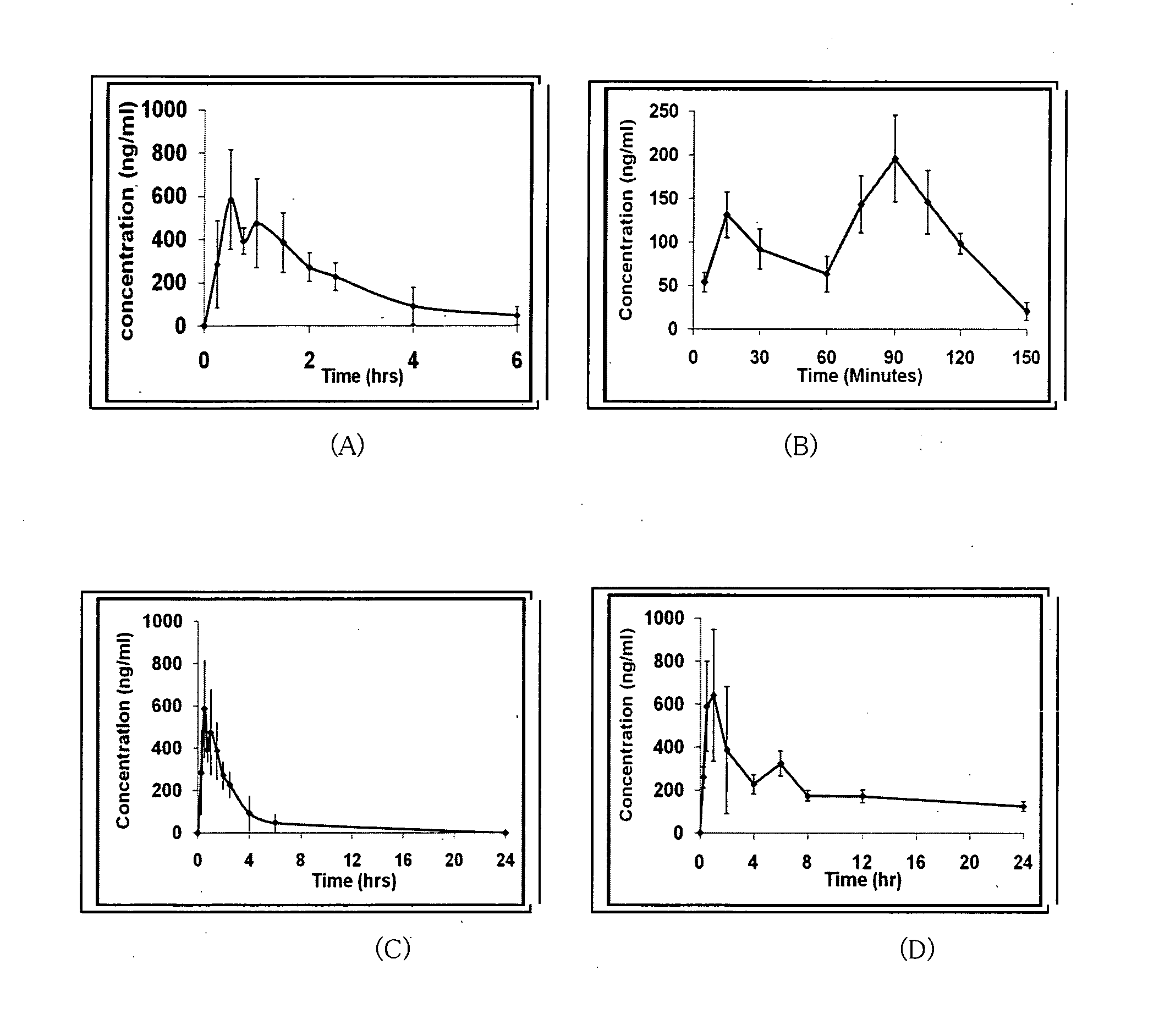
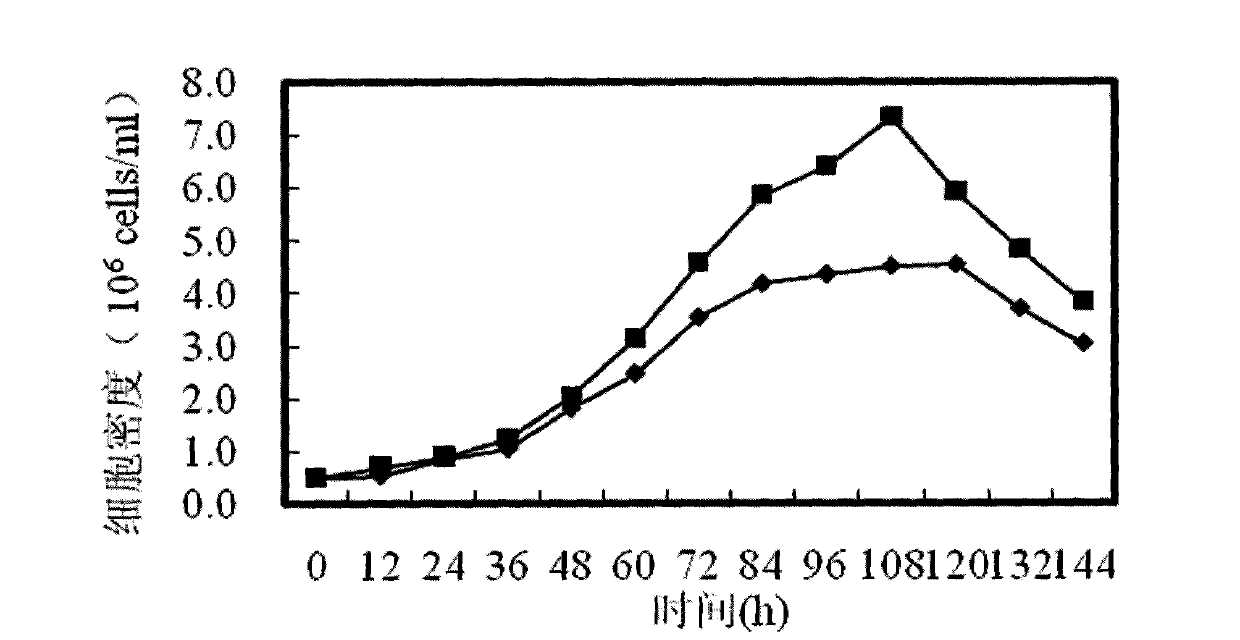
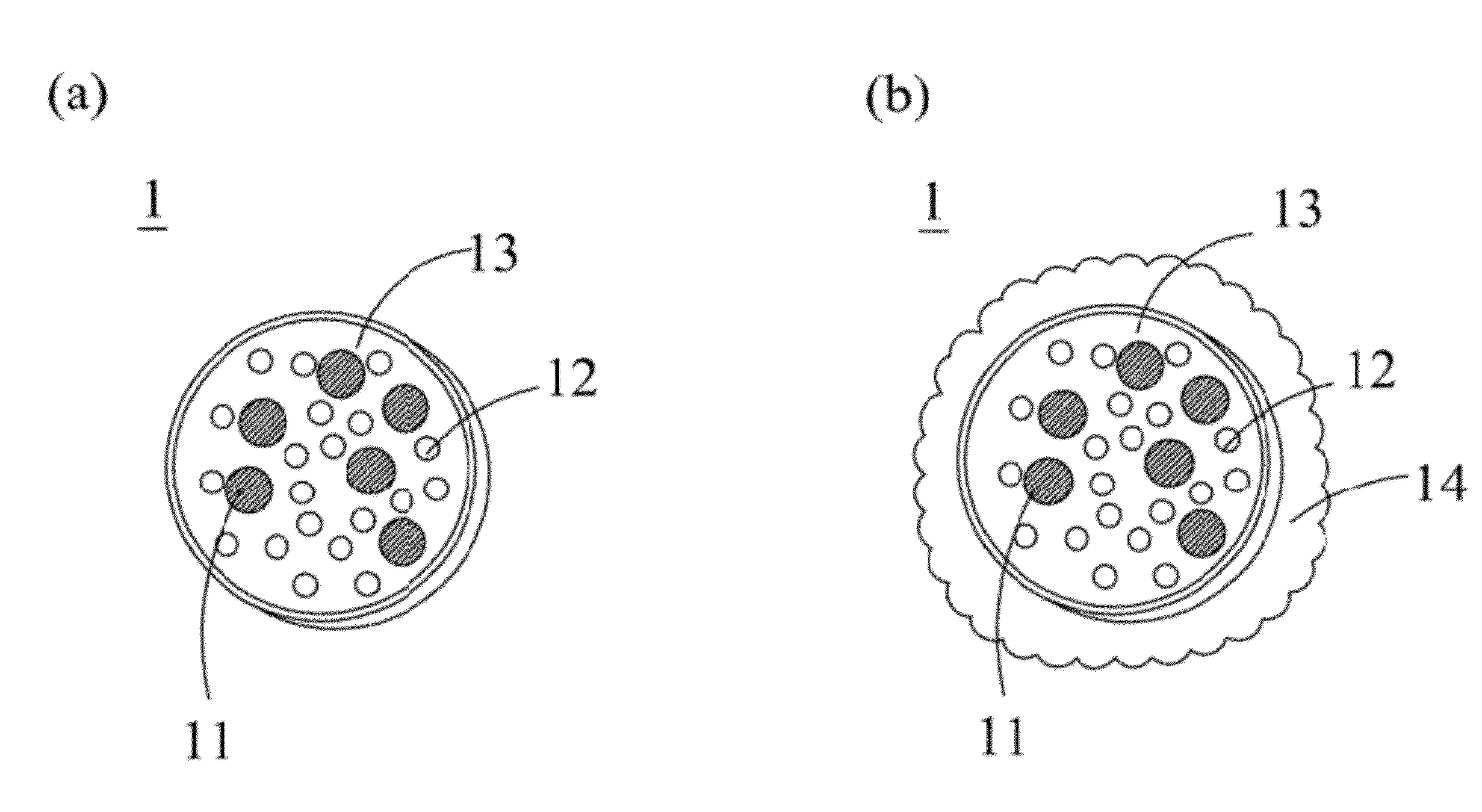
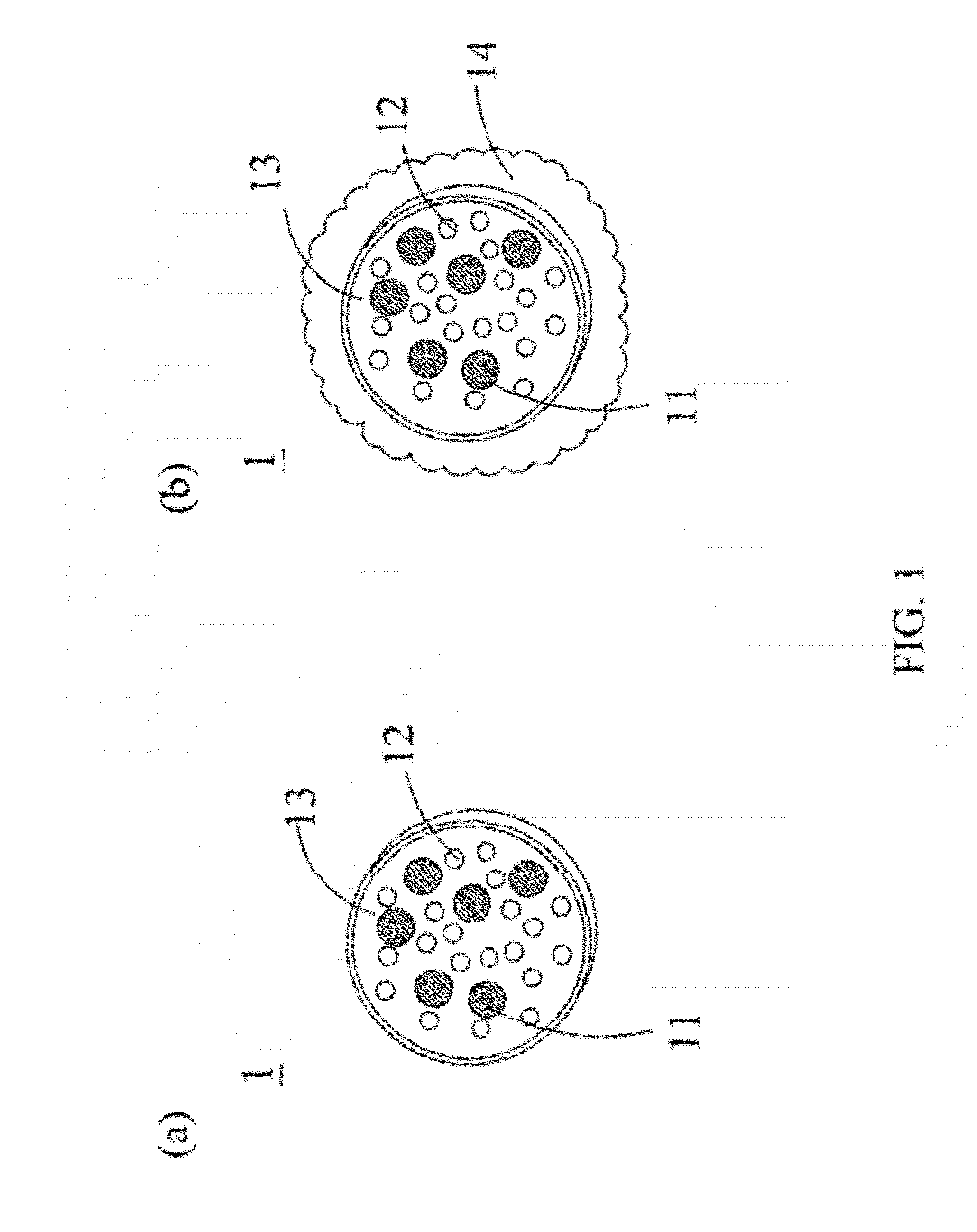
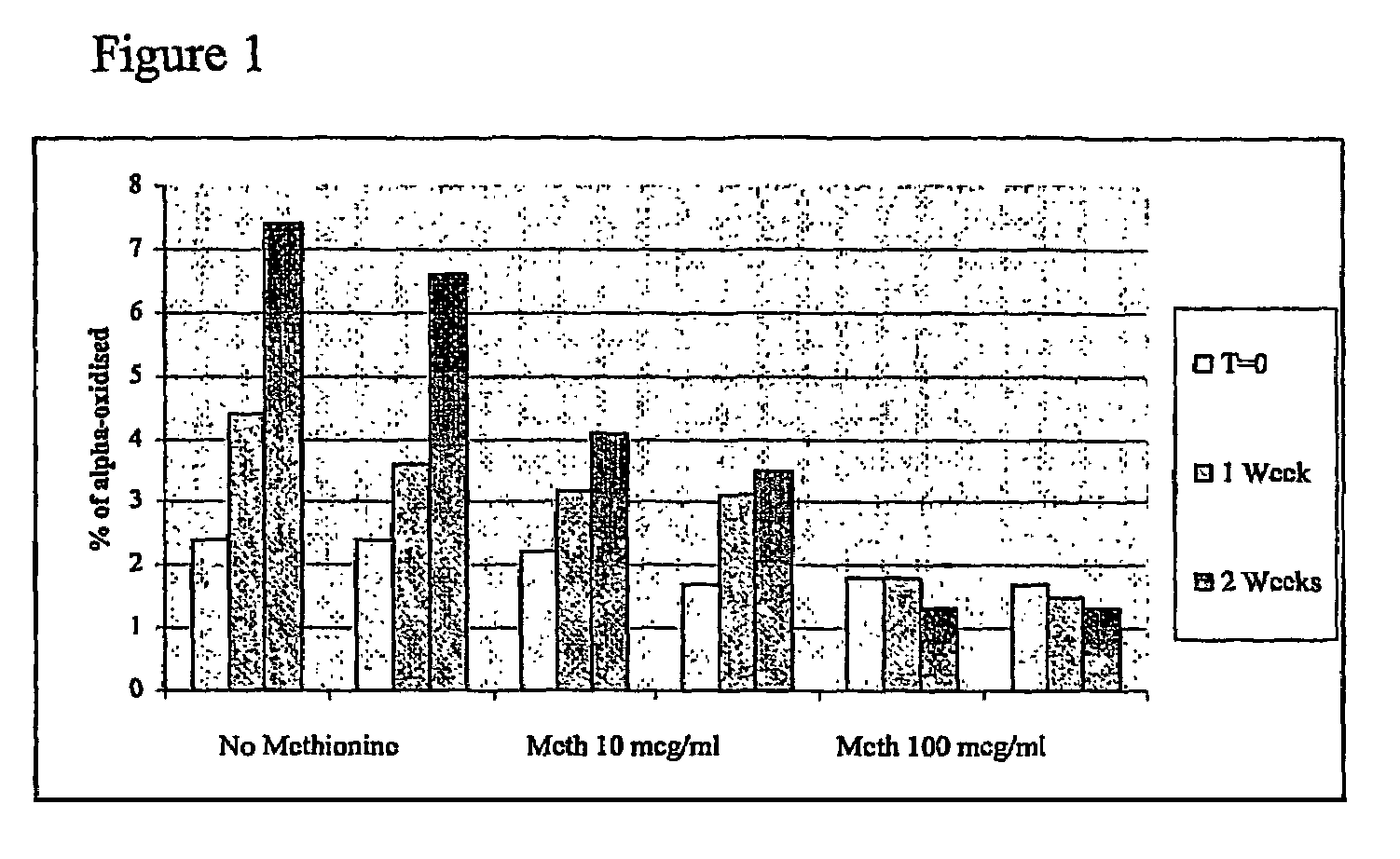
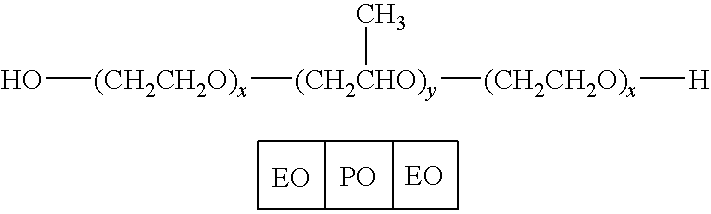
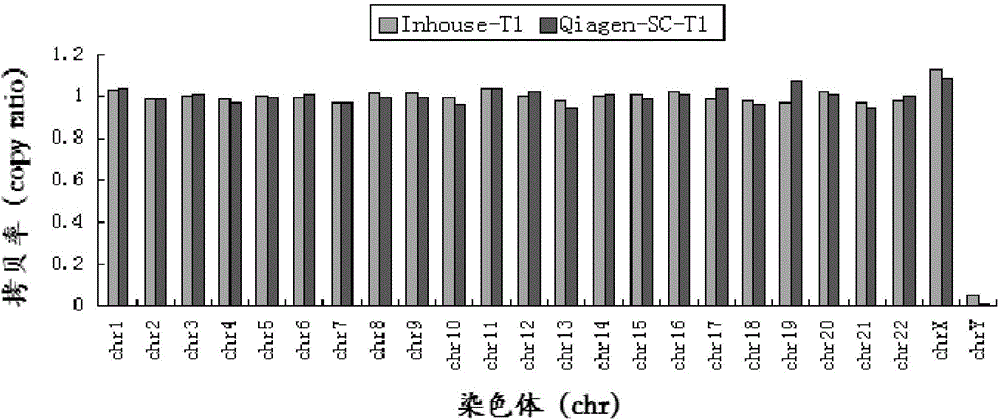
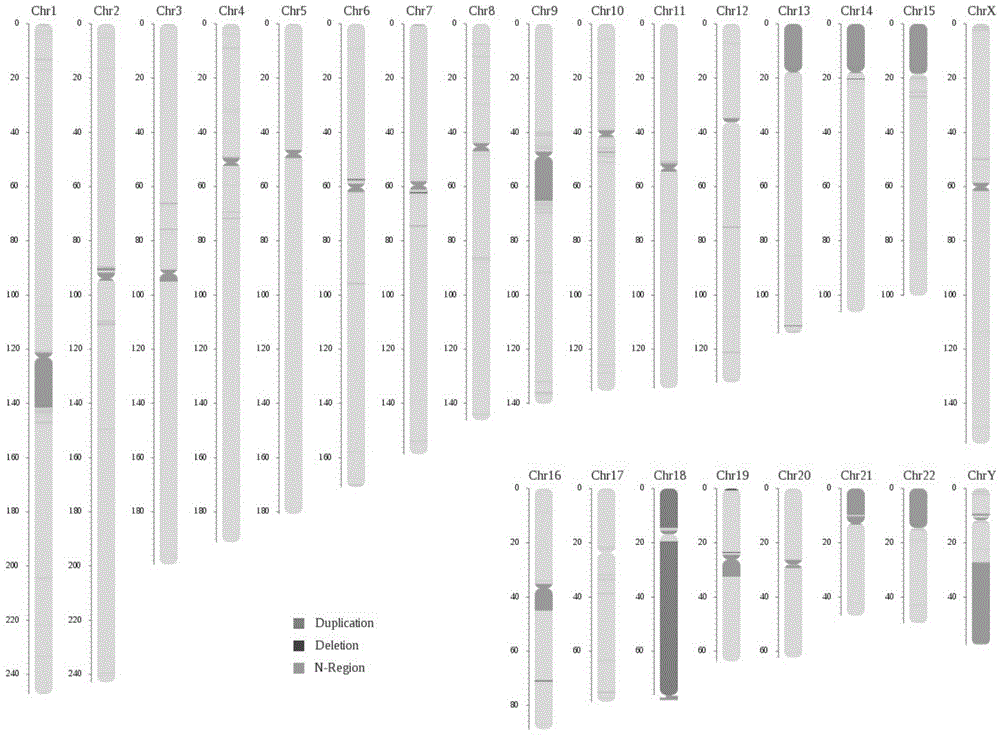
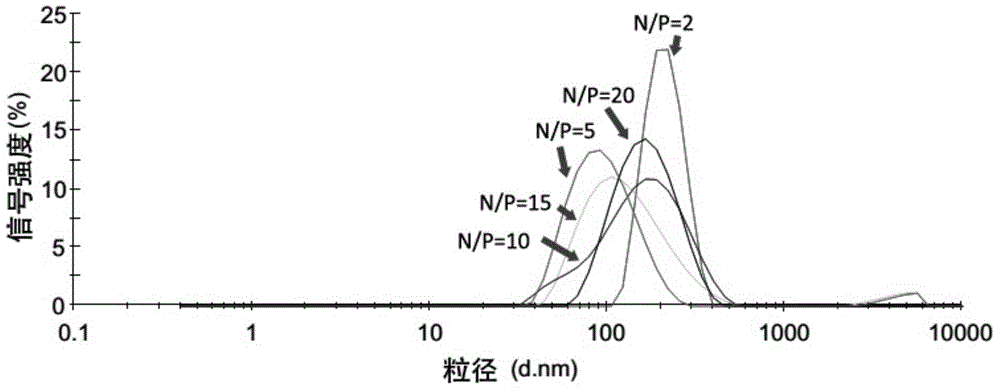
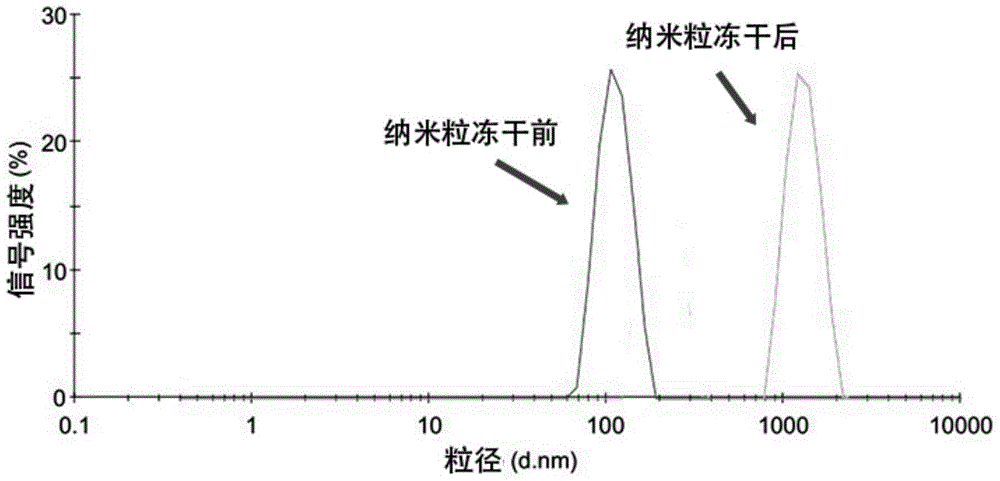
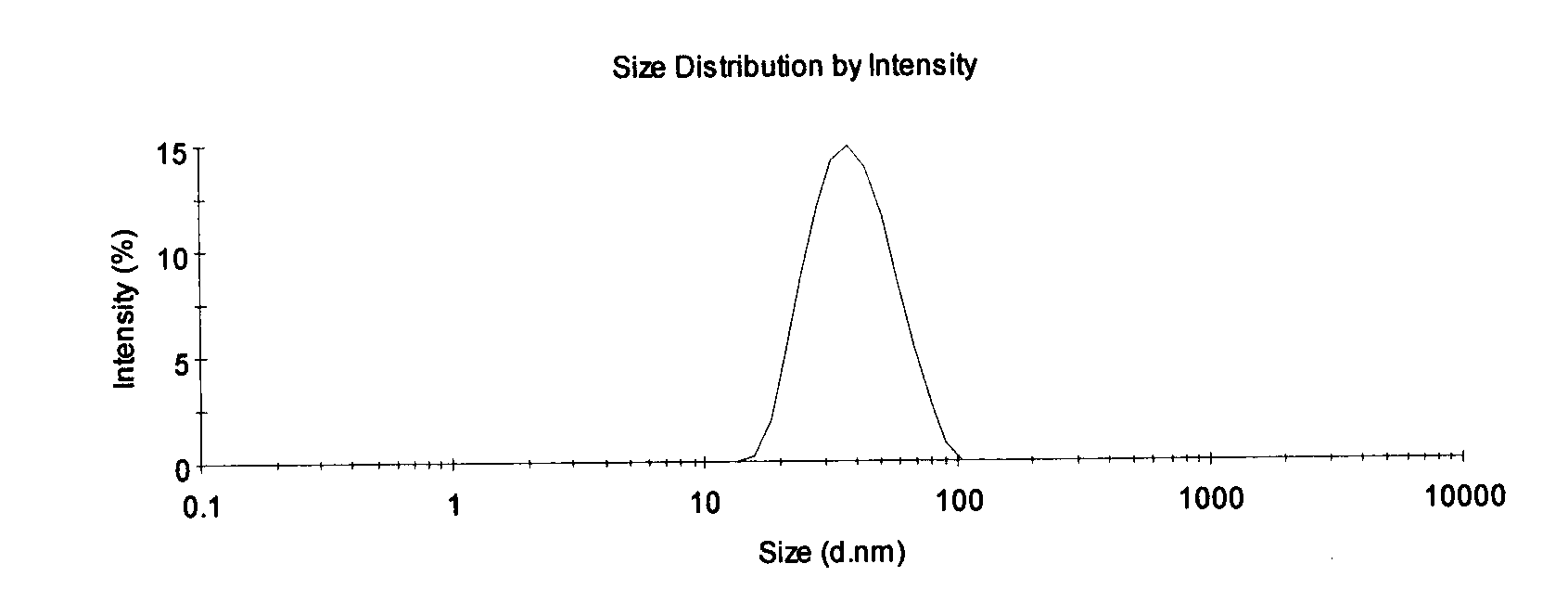
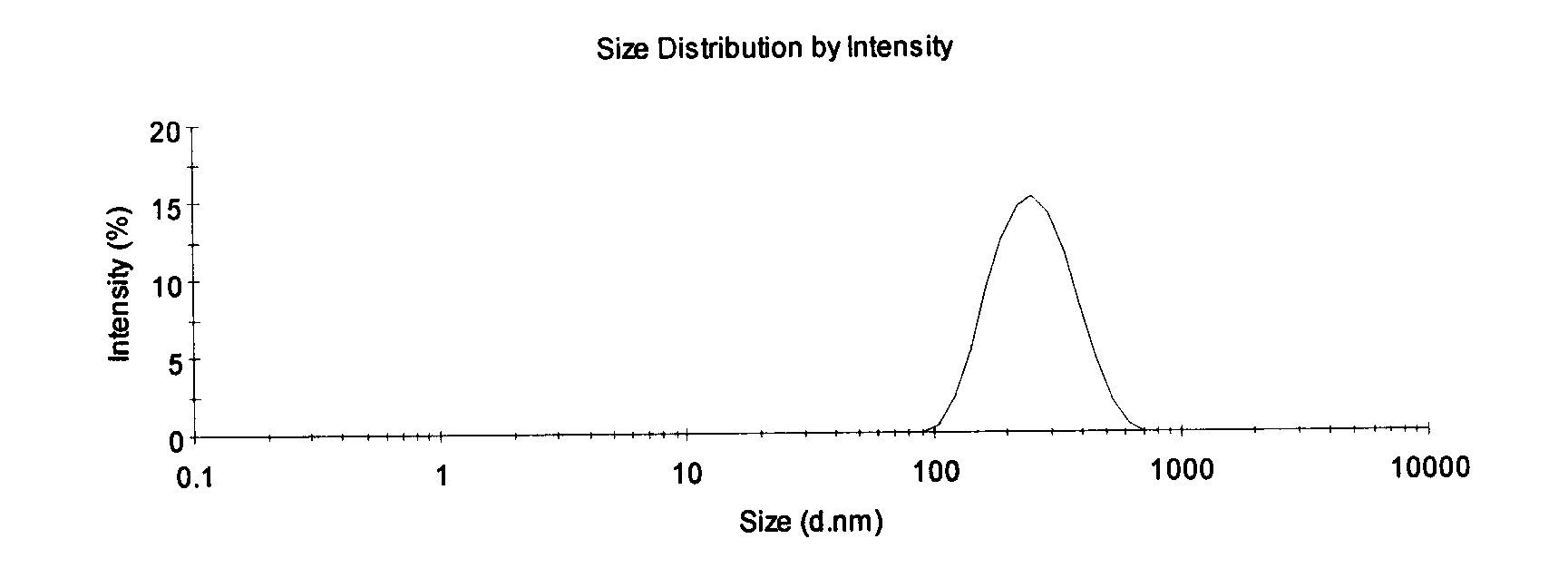
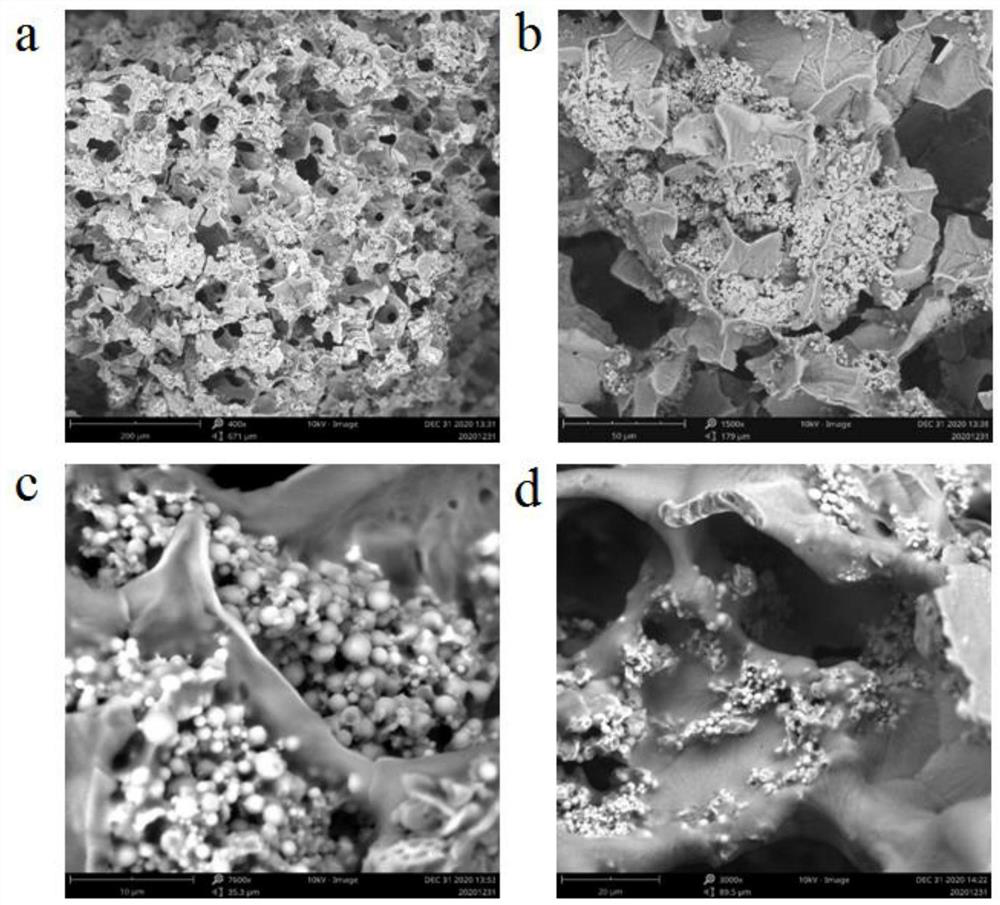
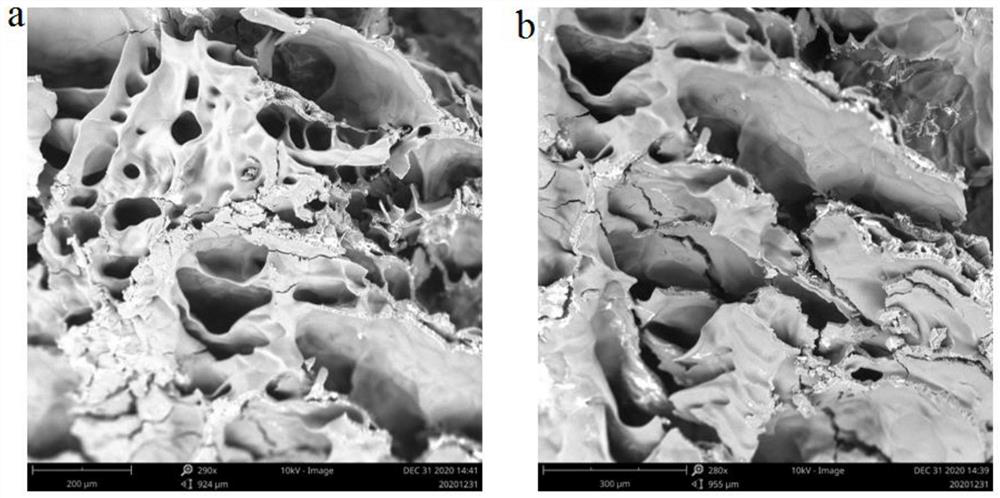
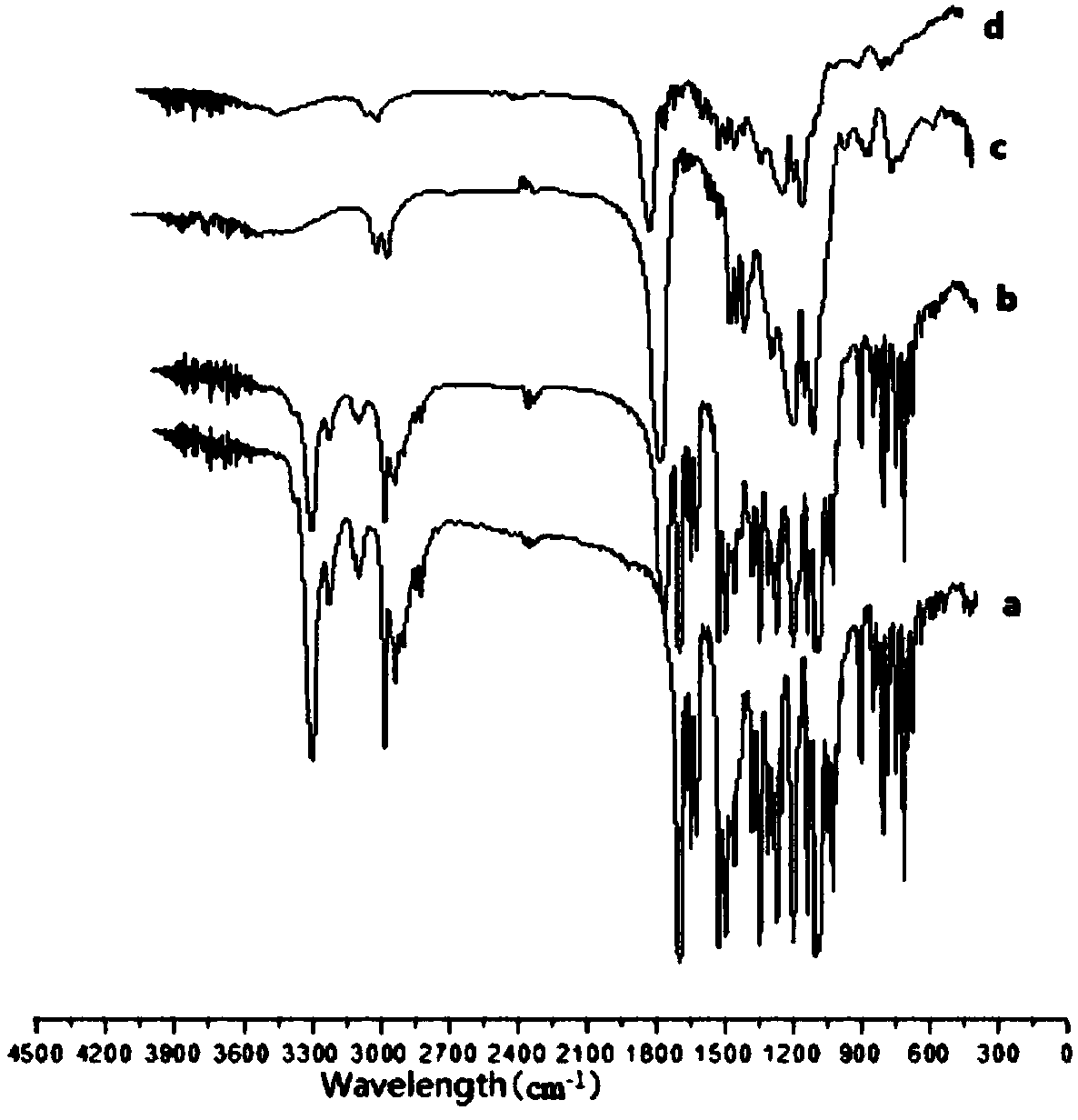
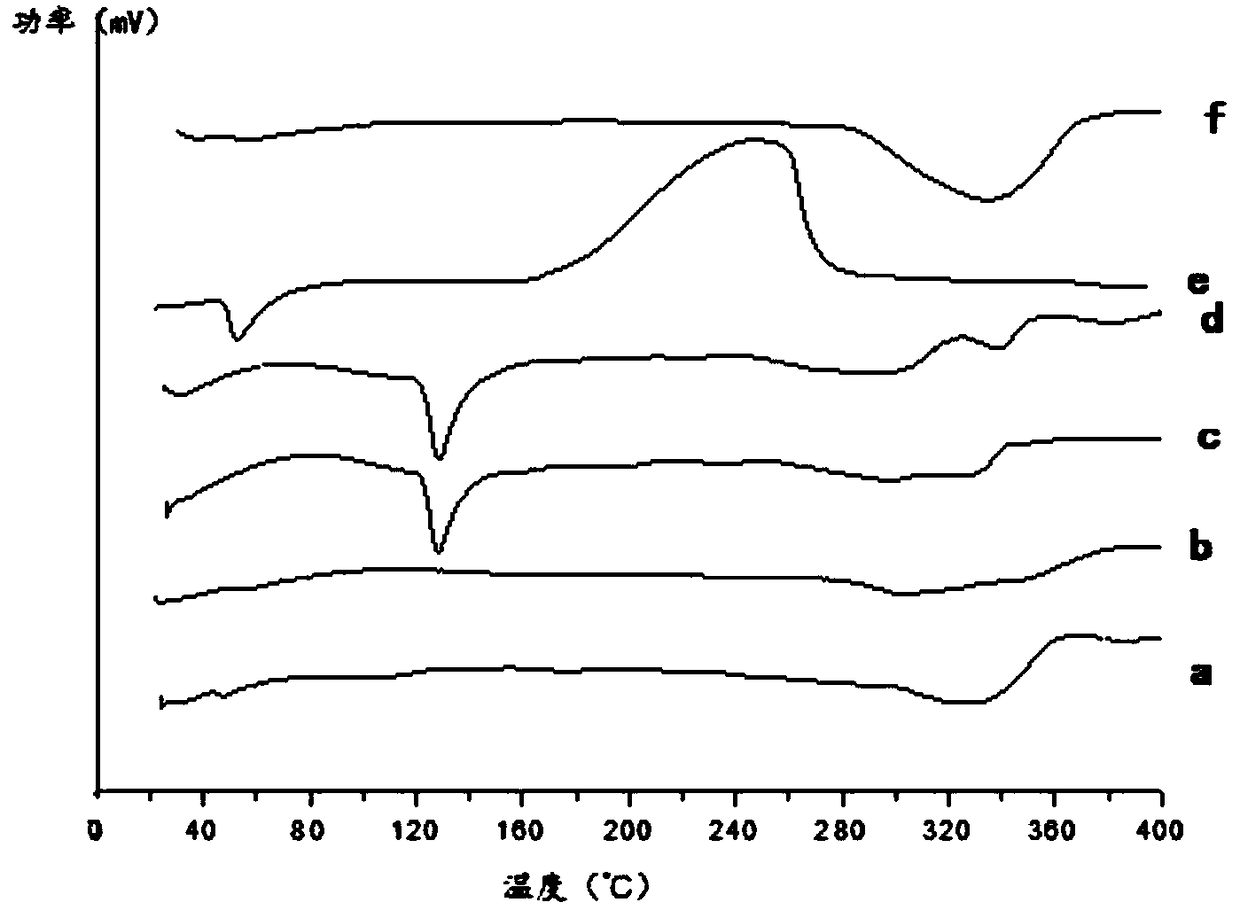
Patents
Literature
31 results about "Pluronic F68" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
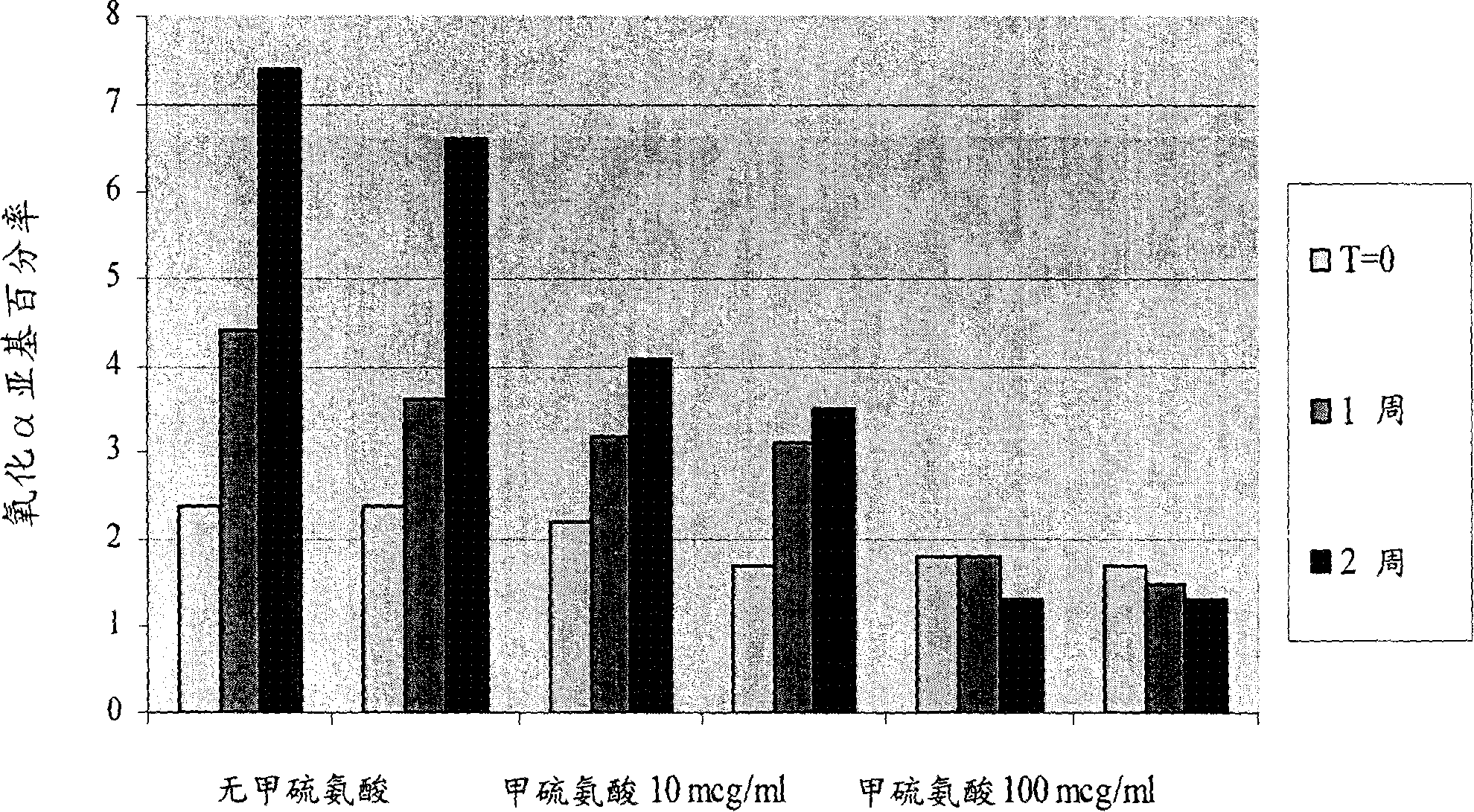
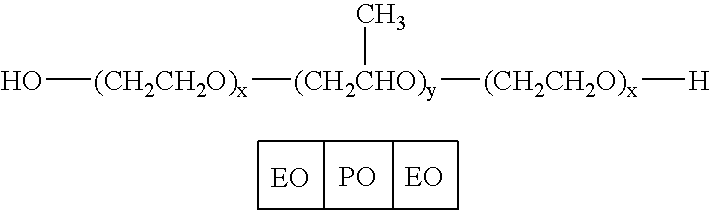
Pluronic F68 is used in cell culture as a stabilizer of cell membranes protecting from membrane shearing and additionally acts as an anti-foaming agent.
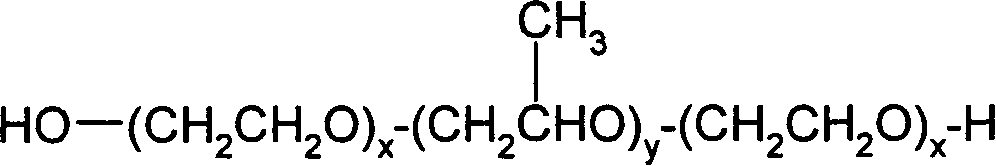
Liquid pharmaceutical formulations of fsh and lh together with a non-ionic surfactant
ActiveCN1795012APeptide/protein ingredientsPharmaceutical non-active ingredientsFollicle-stimulating hormoneNon ionic
The present invention relates to the field of pharmaceutical preparations of follicle stimulating hormone (FSH), luteinizing hormone (LH) and mixtures of FSH and LH and methods for the production of these preparations. The present invention also provides liquid or lyophilized formulations of FSH, or LH, or FSH and LH, which contain a surfactant selected from Pluronic® F77, Pluronic F87, Pluronic F88 and Pluronic F68.
Owner:ARES TRADING SA
Controlled release micro-capsule for osteogenic action
ActiveUS20110305754A1Improve actionHigh drug loadingBiocidePretreated surfacesPluronic F68Controlled release
The present invention relates to a microcapsule for controlled release of flavanoid compound and a process for preparation thereof. The microcapsule comprising a core particle consisting of a calcium salt, Pluronic F68 [poly (ethylene oxide-co-polypropylene co-polypropylene oxide), block poly oxyethylene-polypropylene block copolymer], loaded with a flavanoid compound, the resulting core particle having a plurality of alternate layers of cationic and anionic polyelectrolytes adsorbed thereon and an outer layer formed by a bile salt, wherein the flavanoid is ranging between 10 to 96% by weight.
Owner:COUNCIL OF SCI & IND RES
Chinese hamster ovary culture medium as well as preparation method and application thereof
InactiveCN102021139ACulture regulationCultivate delivery and controlTissue cultureAdditive ingredientCell culture media
The invention discloses a Chinese hamster ovary culture medium as well as a preparation method and application thereof. The culture medium comprises ascorbic acid, pyridoxine hydrochloride, vitamin B12, nicotinamide, riboflavin, thiamine chloride, choline chloride, folic acid, calcium pantothenate, sodium pyruvate, reduced glutathione, inositol, biotin, hypoxanthine, putrescine, lipoic acid, linoleic acid, thymine, glucose, HEPES (2-hydroxyethyl) buffer solution, dextran sulphate, Pluronic-F68, various amino acids and salts thereof and various inorganic salts. The culture medium has low cost, and the component does not contain animal origin ingredients, does not contain protein and has definite chemical ingredients. All ingredients are cooperated and blended, and the Chinese hamster ovary culture medium can satisfy the basic growth requirement of cells, and can effectively regulate, transfer and control the culture of cells so as to realize good high-density cell culture. Compared with the culture effect of the existing culture medium, the culture effect of culture medium disclosed in the invention is at least equivalent to even superior to the existing culture medium.
Owner:EAST CHINA UNIV OF SCI & TECH
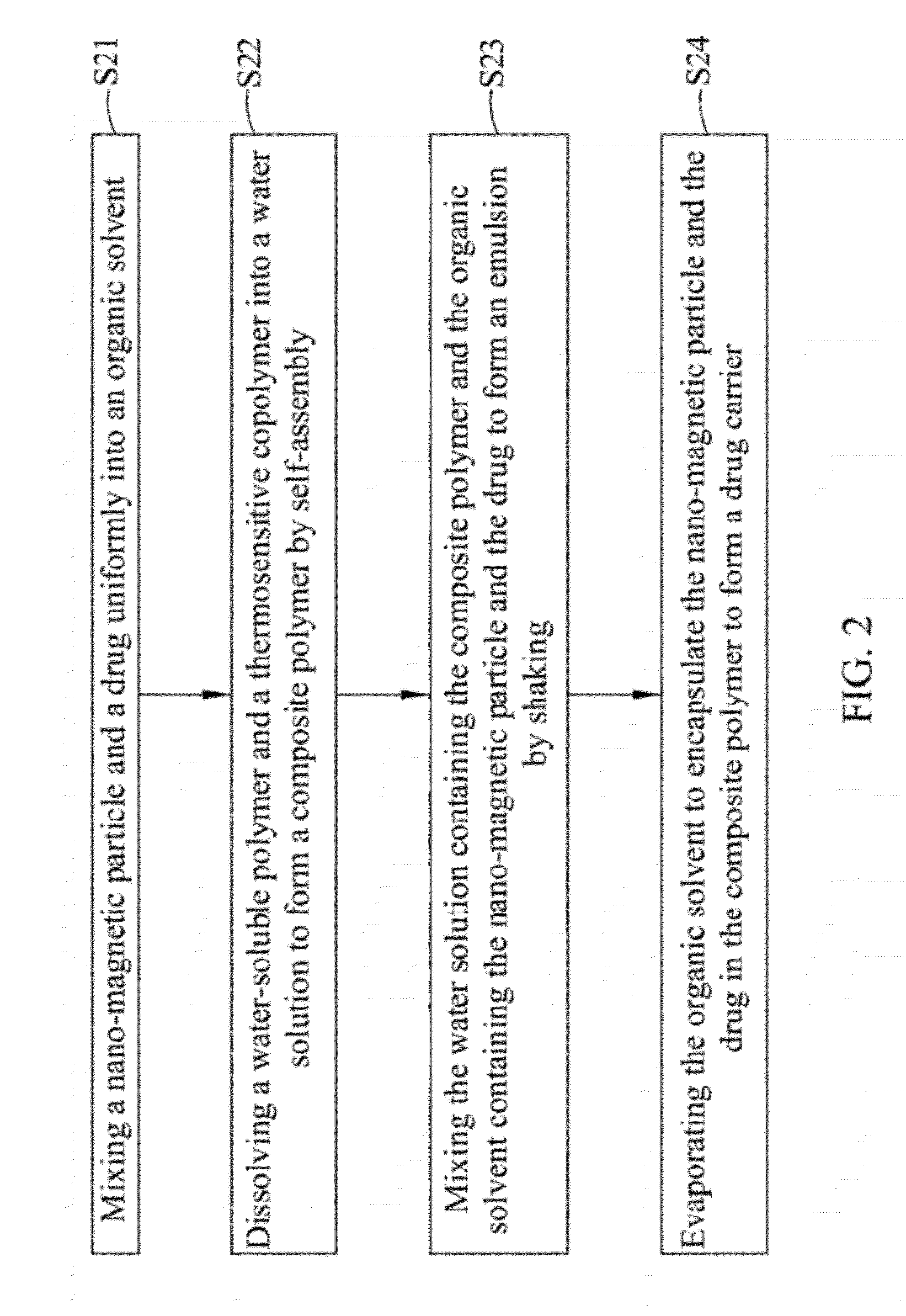
Drug carrier with thermal sensitivity, manufacturing method thereof, and use thereof
InactiveUS20120294806A1Good biocompatibilityHighly sensitive NMR contrast agentPowder deliveryDiagnostic recording/measuringPolyvinyl alcoholDrug release
A drug carrier with thermal sensitivity, a manufacturing method thereof, and a use thereof are disclosed. The drug carrier comprises a nano-magnetic particle, a drug, a composite polymer, and a dense silica shell. The nano-magnetic particle and the drug are encapsulated in the composite polymer which is formed by self-assembly a water-soluble polymer (such as poly vinyl alcohol) and a thermosensitive copolymer (such as Pluronic F68 or Pluronic F127). The stability and drug release of the drug carrier a can be adjusted by combining PVA and thermosensitive copolymer with a different ratio. When an external magnetic field was applied, the cores exhibit significant size shrinkage and the diameter of the drug carrier decreases more than 10 folds due to the change of temperature, which causes burst-like drug release because of shell destruction and physical collapse of the drug carrier.
Owner:NAT CHIAO TUNG UNIV
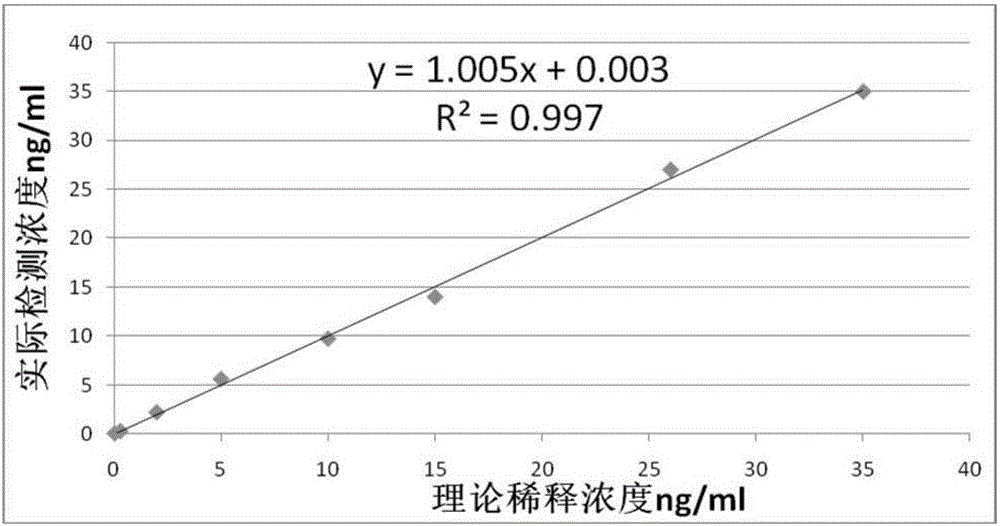
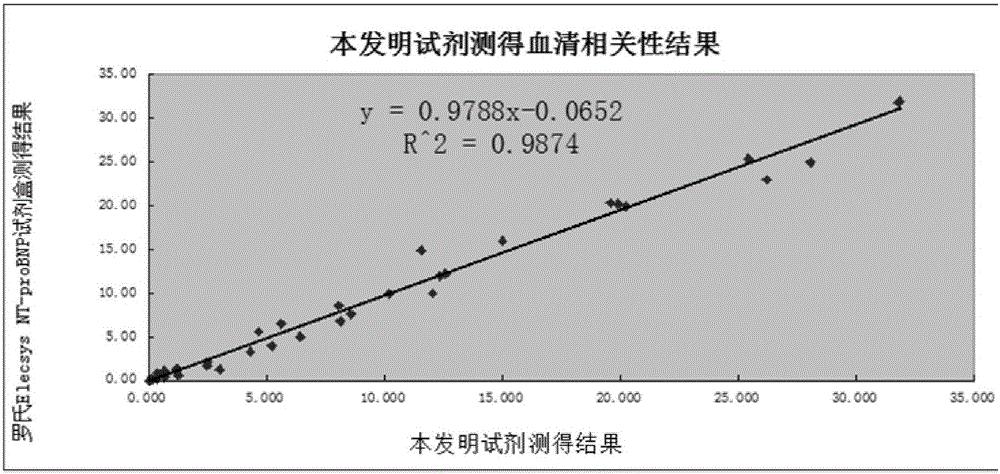
NT-proBNP fluorescence immunoassay reagent and preparing method thereof
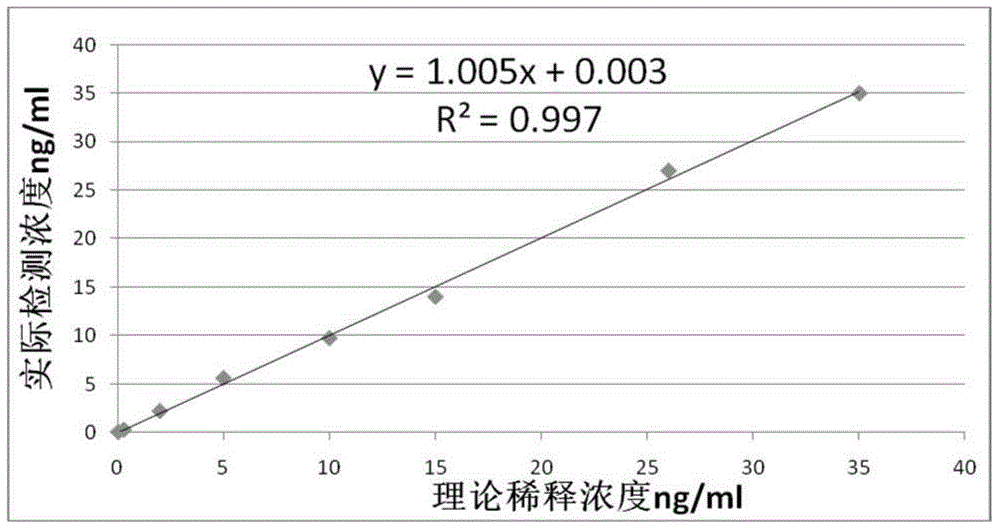
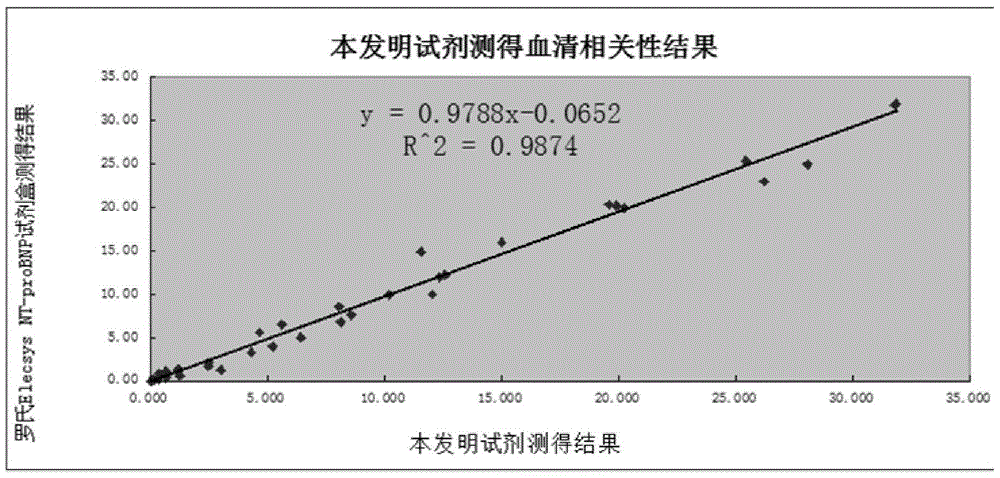
ActiveCN105092832AThe composition formula is reasonableHigh sensitivityMaterial analysisSerum igePluronic F68
The invention relates to an NT-proBNP fluorescence immunoassay reagent and a preparing method thereof, which belong to the technical field of medical detection. The NT-proBNP fluorescence immunoassay reagent comprises an NT-proBNIP antibody for marking, a goat anti-chicken IgY antibody for marking, an NT-proBNP2 antibody for coating, a chicken IgY antibody for coating, a particle resuspension and a sample pad conditioning fluid, wherein the particle heavy suspension is Tris-HCl heavy suspension with 100-200mM Tris (containing 250-500mu.g / ml mouse IgG, 1-5% of calf serum, 0.1-0.5% of Pluronic F68, 0.5-1% of casein, 0.9% of NaCl, 0.05% of NaN3) and has a pH being 8-9, the sample pad conditioning fluid contains 10mM PBS (containing 0.1-0.5mg / ml of phytolectin) and has a pH being 7-8. The invention specifically discloses a preparing method of the NT-proBNP fluorescence immunoassay reagent, comprising specific particle marking, sample pad pretreatment, antibody coating on an NC film, and test paper assembling. The NT-proBNP fluorescence immunoassay reagent is high in sensitivity, wide in application range and capable of reducing interference in a serum test, and has a good interception effect on red cells.
Owner:NINGBO RUI BIO TECH
Liquid pharmaceutical formulations of fsh and lh together with a non-ionic surfactant
The invention relates to the field of pharmaceutical formulations of follicle-stimulating hormone (FSH), luteinising hormone (LH), and mixtures of FSH and luteinising hormone (LH), and to methods of producing such formulations. The invention provides a liquid or freeze-dried formulation of FSH, or LH, or FSH and LH comprising a surfactant selected from Pluronic® F77, Pluronic F87, Pluronic F88 and Pluronic F68.
Owner:ARES TRADING SA
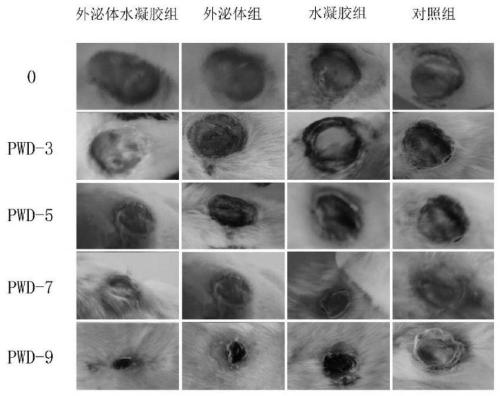
Exosome hydrogel wound dressing and preparation method thereof
The invention belongs to the technical field of biomedicine, and particularly relates to an exosome hydrogel wound dressing and a preparation method thereof. The exosome hydrogel wound dressing comprises mesenchymal stem cell exosomes and hydrogel, and the hydrogel is composed of pluronic F127 and pluronic F68. The preparation method comprises the following steps: respectively preparing physiological saline solutions of pluronic F127 and pluronic F68 and mesenchymal stem cell exosome physiological saline resuspension, and mixing the solutions at 4 DEG C to obtain the exosome hydrogel wound dressing. Experimental results show that compared with the mode that mesenchymal stem cell exosomes and hydrogel are independently adopted, the wound dressing prepared through the method can better promote regeneration of skin wound cells and shorten the wound healing time.
Owner:JILIN UNIV +2
Antibacterial peptide preservative, antibacterial peptide preparation containing the same, and preparation method for the antibacterial peptide preparation
ActiveCN102205128ANo reduction in biological activityImprove or maintain stabilityAntibacterial agentsPeptide/protein ingredientsArginineAntioxidant
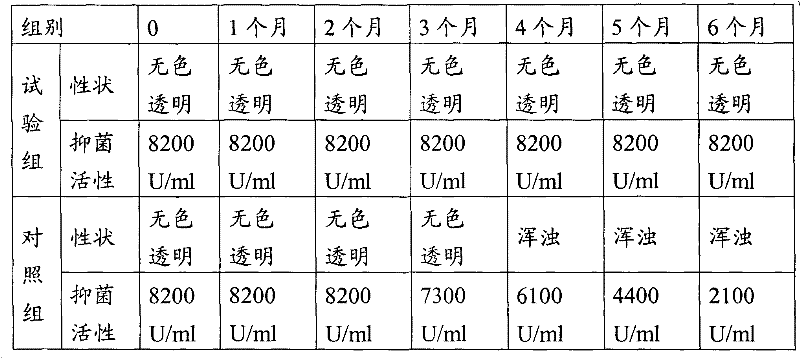
The invention discloses an antibacterial peptide preservative, an antibacterial peptide preparation containing the same, and a preparation method for the antibacterial peptide preparation, and belongs to the field of pharmaceutical preparation. The antibacterial peptide preservative comprises a buffer, an amino acid, a stabilizing agent and an antioxidant. The antibacterial peptide preparation comprises the antibacterial peptide, a buffer, an amino acid, a stabilizing agent and an antioxidant. The preparation method for the antibacterial peptide preparation comprises: uniform mixing the antibacterial peptide, the buffer, the amino acid, the stabilizing agent, the antioxidant and water for injection, followed by adjusting pH value of the resulting mixture to 8.0-10.5, wherein the buffer is a Tris buffere, the amino acid is arginine, lysine or glutamic acid, the stabilizing agent is tween-80, Pluronic F68 or Pluronic F88, the antioxidant is methionine. According to the technical scheme provided by the embodiments of the present invention, the antibacterial peptide can be preserved for a long time without biological activity reducing, and preparation technology is simple so as to be suitable for an industrial production.
Owner:QINGDAO VLAND BIOTECH INC +1
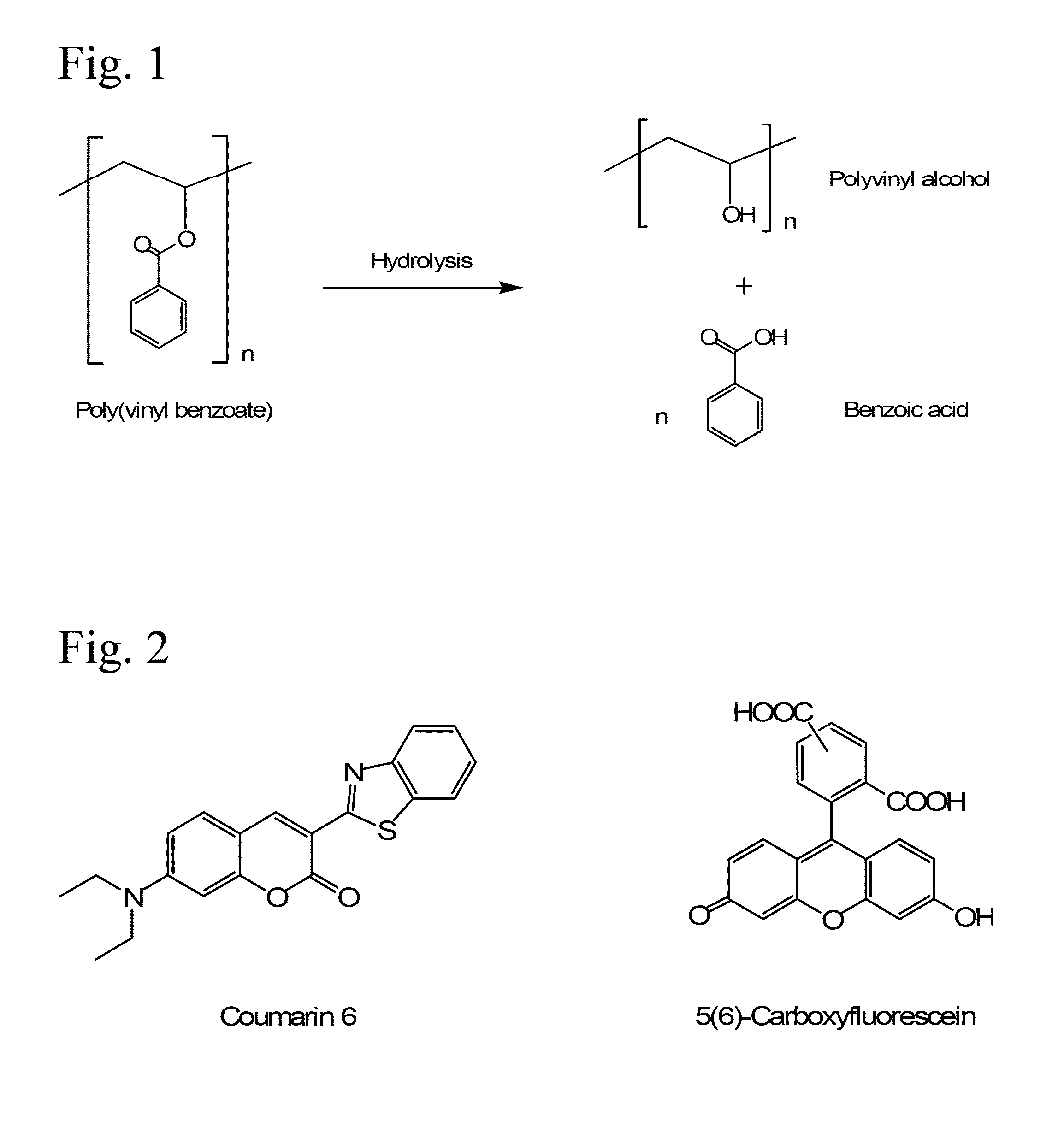
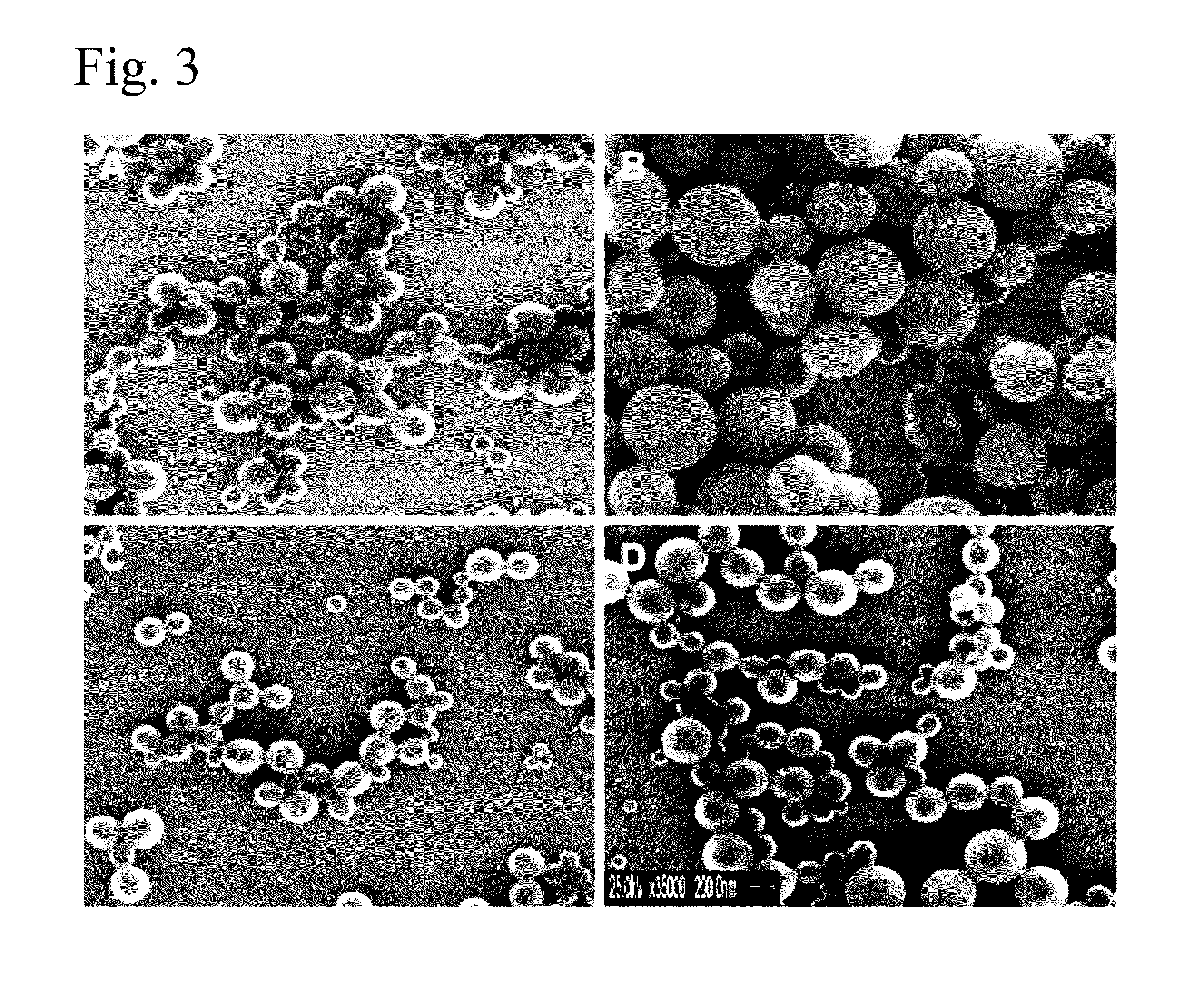
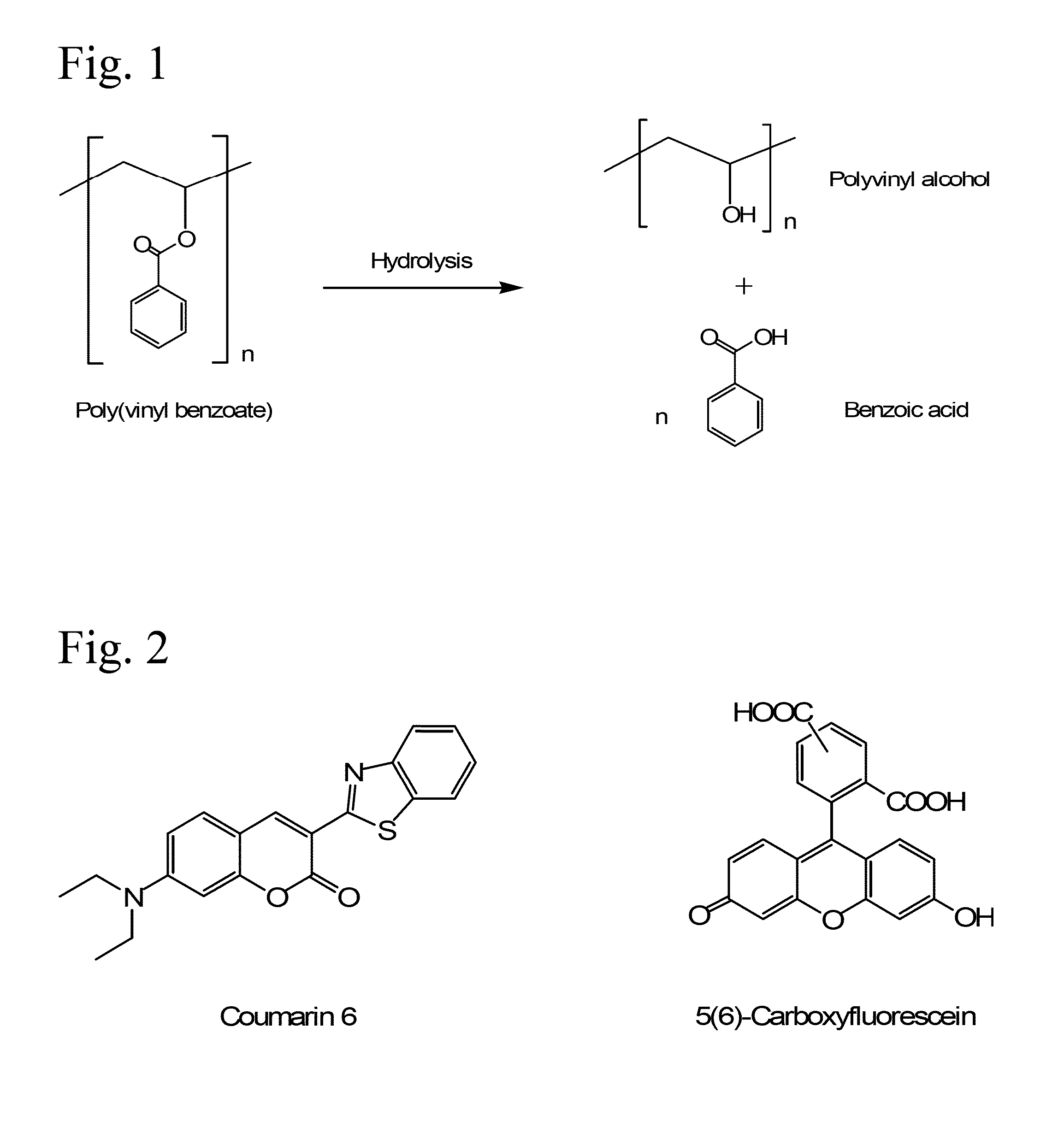
Poly(vinyl benzoate) nanoparticles for molecular delivery
ActiveUS20130243832A1Avoid rapid degradationPowder deliverySolution deliveryBovine aortaBenzoic acid
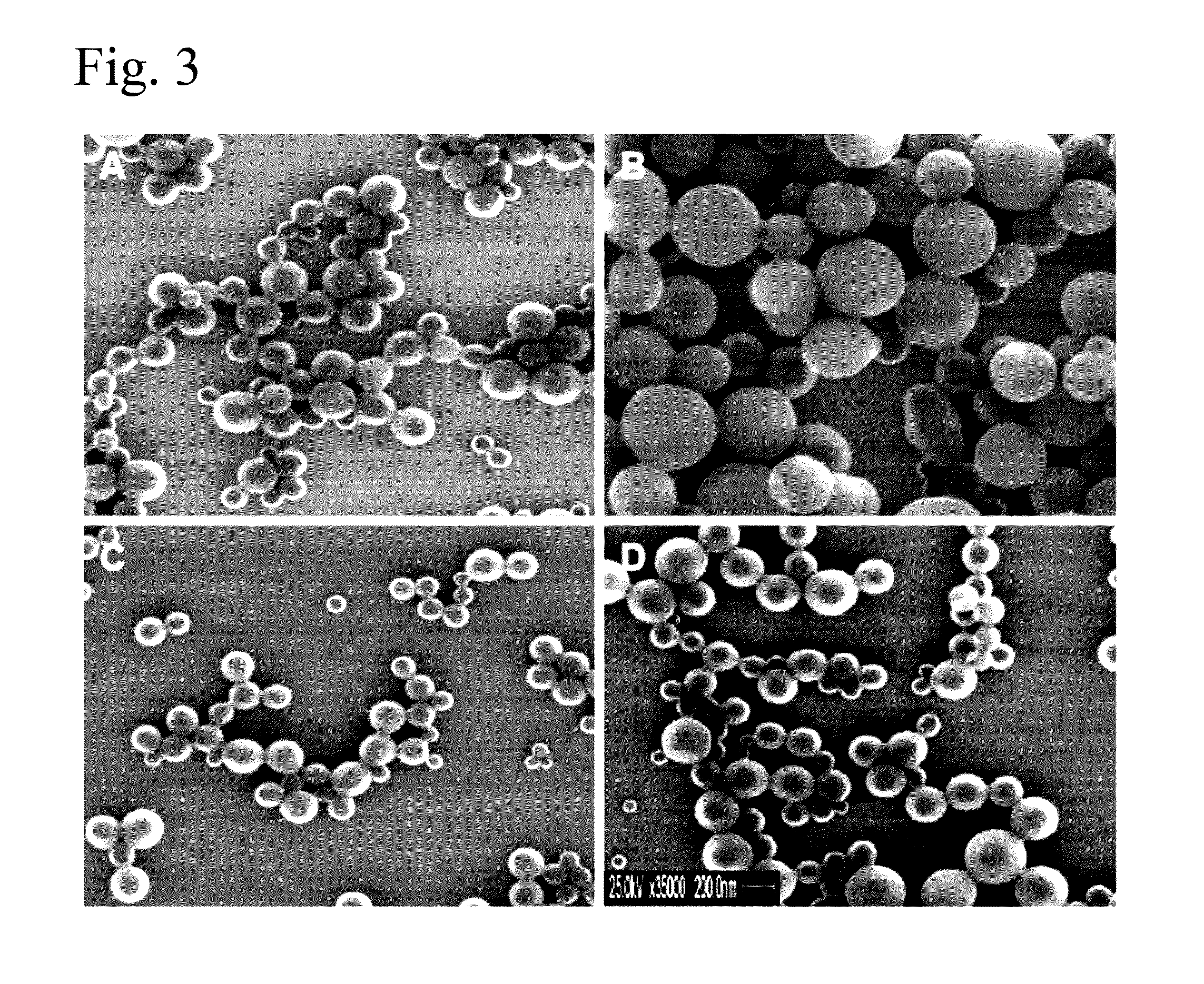
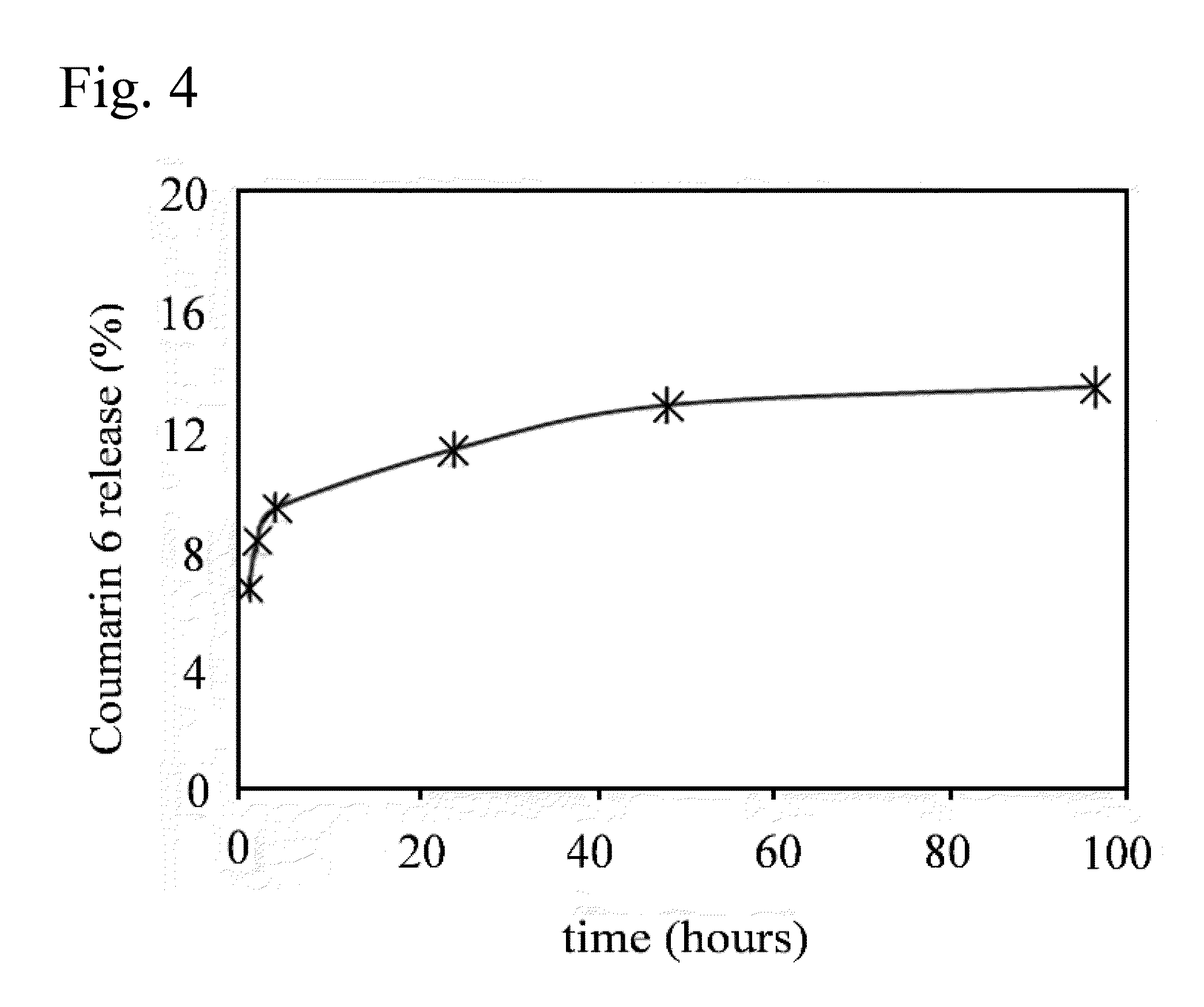
The present invention comprises poly(vinyl benzoate) nanoparticle suspensions as molecular carriers. These nanoparticles can be formed by nanoprecipitation of poly(vinyl benzoate) in water using Pluronic F68 as surfactant, to create spherical nanostructures measuring about 200-250 nm in diameter which are stable in phosphate buffer and blood serum, and only slowly degrade in the presence of esterases. Kinetics experiments in phosphate buffer indicate that 78% of the coumarin-6 was encapsulated within the polymer matrix of the nanoparticle, and the residual 22% of coumarin-6 was surface-bound and quickly released. The nanoparticles are non-toxic in vitro towards human epithelial cells (IC50>1000 μg / mL) and primary bovine primary aortic endothelial cells (IC50>500 μg / mL), and exert non-observable bactericidal activity against a selection of representative test microbes (MIC >250 μg / mL). Poly(vinyl benzoate) nanoparticles are suitable carriers for molecular delivery of lipophilic small molecules such as drugs pharmaceutical and imaging agents.
Owner:UNIV OF SOUTH FLORIDA
Liquid pharmaceutical formulations of FSH and LH together with a non-ionic surfactant
The invention relates to the field of pharmaceutical formulations of follicle-stimulating hormone (FSH), luteinising hormone (LH), and mixtures of FSH and luteinising hormone (LH), and to methods of producing such formulations. The invention provides a liquid or freeze-dried formulation of FSH, or LH, or FSH and LH comprising a surfactant selected from Pluronic® F77, Pluronic F87, Pluronic F88 and Pluronic F68.
Owner:ARES TRADING SA
Kit for single cell genome-wide amplification and application thereof
InactiveCN105483115ALow priceEfficient and stable amplification effectMicrobiological testing/measurementDNA preparationPluronic F68Genetics
The invention discloses a kit for single cell genome-wide amplification and application thereof. The invention protects a kit for single cell genome amplification first, comprising a second amplification system; the second amplification system is composed of 10*Phi29buffer, water and a first amplification system; the first single amplification system is composed of dNTP (1.8-2.2)*10-8 mol, N8 primers (1.8-2.2)*10-11 mol in total concentration, BSA (4.5-5.5)*10-3 mg, Pluronic F68 0.0045-0.0055Mul, and DNA polymerase 9-11 U Phi29; the N8 primers are random primers, composed of eight deoxyribonucleotides, in a random arrangement of A, G, C and T. The WGA kit low in cost, high in efficiency and stable in amplification effect is developed based on the multiple exchange amplification principle and amplification features of DNA polymerase Phi29, and the kit has an important applicable prospect and polarization value.
Owner:MGI TECH CO LTD
A kind of nt-probnp fluorescent immunological reagent and preparation method thereof
ActiveCN105092832BThe composition formula is reasonableHigh sensitivityMaterial analysisSerum igePluronic F68
Owner:NINGBO RUI BIO TECH
Preparation technology and application of paeoniflorin wheat gliadin nanoparticles
InactiveCN106265599APromote absorptionEasy to obtainPowder deliveryOrganic active ingredientsPluronic F68Alcohol
Owner:苏州承瑞健康科技有限公司
Allicin fatty milk injection and preparation technics thereof
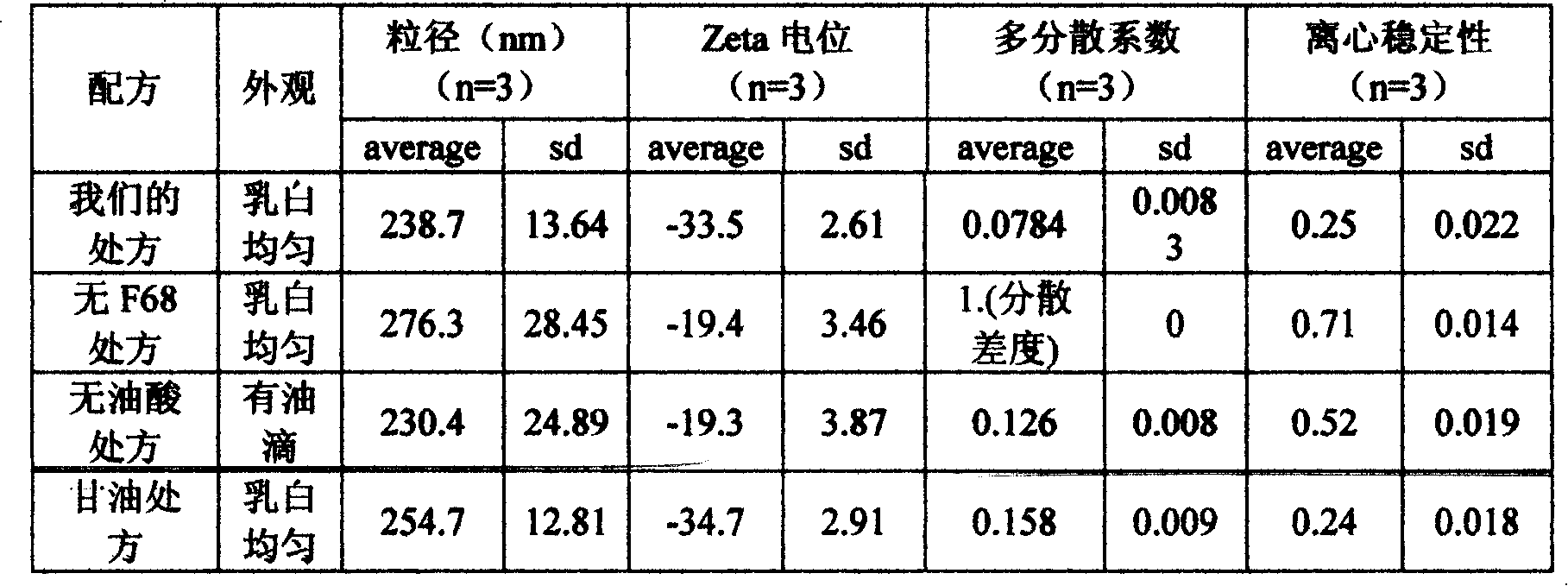
InactiveCN101112360AReduce osmotic pressureQuality cannot be guaranteedSulfur/selenium/tellurium active ingredientsAntiviralsYolkZeta potential
The invention discloses an allicin fat emulsion injection and a preparation process thereof; the invention is the drug which is made by the materials with the following mix ratios by weight, while the pH value of which is 6.5 to 7.5, the average particle size of the emulsion particles is 200nm, and the particle size thereof is 100 to 300nm and the Zeta potential is minus 50 to 80mv. The raw materials are: allicin, egg yolk lecithin, pluronic F68, oleic acid, and injection water and so on. The preparation process is that the egg yolk lecithin, oleic acid, vitamin E and soya bean oil are taken at first under the aseptic conditions for mixing, heating, agitating and dissolving, then the allicin is added to agitate till dissolution, and the filtrate can be obtained after the pressure filtration by a microporous membrane; the glycerol is taken at first and the injection water, disodium ethylene diamine tetraacetate and pluronic F68 are added for mixing and agitating till the dissolution, then the filtrate can be obtained after the pressure filtration by the microporous membrane; after the mixture of the two filtrates, a high speed agitator is used for agitating, the product is finally obtained through a high pressure homogenizer. The invention is characterized by no irritating effect and good stability, etc.
Owner:福州璐珈医药科技有限公司
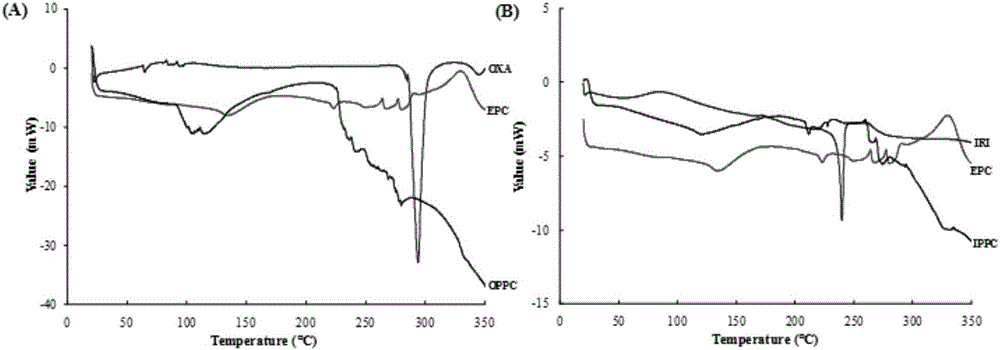
Oxaliplatin-and-irinotecan jointly loading lipid emulsion and preparing method thereof
ActiveCN106619509AImprove oil-water partition coefficientImprove encapsulationOrganic active ingredientsOrganic non-active ingredientsLipid formationPluronic F68
The invention discloses an oxaliplatin-and-irinotecan jointly loading lipid emulsion prepared based on the drug lipid composite technology. The oxaliplatin-and-irinotecan jointly loading lipid emulsion is prepared from oxaliplatin with the concentration of 0.5 mg / ml-2 mg / ml, irinotecan with the concentration of 0.5 mg / ml-5 mg / ml, 5%-20% of injection oil, 0.5%-2.5% of phospholipid, 0.1%-2% of pluronic F68, 0.01%-0.5% of a stabilizing agent, 0.1%-2.5% of glycerol and water. By means of the jointly-loading lipid emulsion prepared with the drug lipid composite technology, synchronous releasing of two drugs in the human body can be coordinated, it is guaranteed that the two drugs are kept in the optimized dosage ratio when reaching the tumor site, and therefore the best collaborative effect is achieved.
Owner:SHANDONG UNIV
Nano-scale delivery system loading two surfactants and having anti-tumor drug resistance and preparation method of nano-scale delivery system
ActiveCN104382866AReverse drug resistanceGood water solubilityOrganic active ingredientsPowder deliverySolubilityFreeze-drying
The invention relates to the technical field of medicines, provides a nano-scale delivery system loading two surfactants and having anti-tumor-drug-resistance, and further provides a preparation method of the nano-scale delivery system and application of the nano-scale delivery system in preparation of anti-tumor medicines. Two surfactants loaded in the drug-loaded nano-particles are Pluronic L61 and Pluronic F68, wherein Pluronic L61 has excellent anti-drug-resistance and excellent biocompatibility, has the activity independent of types of chemotherapeutic drugs and tumors and can be widely applied to chemosensitization of multiple tumors and Pluronic F68 has excellent water solubility and safety, is capable of remarkably improving stability of nano-particles in water and guaranteeing in-vivo long circulation of the nano-particles to reach tumor tissues and further has an effect of freeze-drying protection and no extra freeze-drying protective additive needs to be added.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Propofol microemulsion composition
InactiveCN103156810AImprove stabilityImprove securityNervous disorderHydroxy compound active ingredientsPluronic F68Medicine
The invention discloses a propofol microemulsion composition which does not contain phosphatide and fatty acid triglycerides. The propofol microemulsion composition provided by the invention comprises 0.8-1.2 % (w / v) of propofol, 20-25 % (w / v) of Pluronic F 68, 20-30 % (w / v) of propylene glycol and the balance being water. The invention provides the propofol microemulsion composition which can be administered through gastrointestinal tracts, skins, mucous membranes or injection, and the composition has advantageous of simple technology conditions, greatly improved stability and convenient usage, and is easy to realize productization and be clinically accepted.
Owner:刘青松
Allicin fatty milk injection and preparation technics thereof
InactiveCN101112360BReduce osmotic pressureQuality cannot be guaranteedSulfur/selenium/tellurium active ingredientsAntiviralsGlycerolPharmaceutical Substances
The invention discloses an allicin fat emulsion injection and a preparation process thereof; the invention is the drug which is made by the materials with the following mix ratios by weight, while the pH value of which is 6.5 to 7.5, the average particle size of the emulsion particles is 200nm, and the particle size thereof is 100 to 300nm and the Zeta potential is minus 50 to 80mv. The raw materials are: allicin, egg yolk lecithin, pluronic F68, oleic acid, and injection water and so on. The preparation process is that the egg yolk lecithin, oleic acid, vitamin E and soya bean oil are taken at first under the aseptic conditions for mixing, heating, agitating and dissolving, then the allicin is added to agitate till dissolution, and the filtrate can be obtained after the pressure filtration by a microporous membrane; the glycerol is taken at first and the injection water, disodium ethylene diamine tetraacetate and pluronic F68 are added for mixing and agitating till the dissolution, then the filtrate can be obtained after the pressure filtration by the microporous membrane; after the mixture of the two filtrates, a high speed agitator is used for agitating, the product is finally obtained through a high pressure homogenizer. The invention is characterized by no irritating effect and good stability, etc.
Owner:福州璐珈医药科技有限公司
Wound dressing based on intelligent response type hydrogel-composite microspheres and preparation method
InactiveCN113244444ARegulate immune response processReduced burst effectMicrocapsulesBandagesWound dressingMicrosphere
The invention discloses a wound dressing based on intelligent response type hydrogel-composite microspheres. The wound dressing is hydrogel prepared from intelligent response type hydrogel and composite microspheres, and the mass ratio of the composite microspheres to the intelligent response type hydrogel is (1: 100)-(1: 200); the intelligent response type hydrogel is a composite aqueous solution containing pluronic F68 and pluronic F127; and the composite microsphere comprises a nano-scale sodium alginate single-layer microsphere loaded with a vascular endothelial growth factor (VEGF) and a nano-scale sodium alginate single-layer microsphere loaded with leptin (LP). The wound dressing based on the intelligent response type hydrogel-composite microspheres can be applied to treatment of combined wound, and two medicines with different effects are used, so that the prepared dressing has the dual effects of regulating and controlling the immunoreaction process and promoting angiogenesis.
Owner:JILIN UNIV
Nimodipine long-acting oral suspension liquid and preparation method thereof
ActiveCN108403629AHigh encapsulation efficiencyHigh drug loadingOrganic active ingredientsSenses disorderOral suspensionsNimodipine
The invention discloses nimodipine long-acting oral suspension liquid and a preparation method thereof. The nimodipine long-acting oral suspension liquid is prepared from the following components in parts by weight: 0.1 to 0.3 part of nimodipine nano-particle, 0.1 to 0.3 part of nimodipine, 0.1 to 2 parts of sweetening agent, 1 to 5 parts of pH buffering agent, 0.1 to 2 parts of preservative and 90 to 100 parts of solvent. A carrier material of the nimodipine nano-particle is a combinant of polylactic acid-hydroxyacetic acid copolymer and pluronic F68, and the pluronic F68 is 5 to 40 percent by mass of the carrier material. The nimodipine long-acting oral suspension liquid disclosed by the invention is convenient to orally take and has a good taste; a part of medicines exists in a free state, so that absorption speed can be accelerated and an effect takes quickly; a part of medicines are carried in the polylactic acid-hydroxyacetic acid copolymer and pluronic F68 nano-particles, so that a first pass effect can be reduced, a medicine utilization ratio is high, the drug load of the nano-particles is large, an entrapment ratio is high, release is slow, the acting time is long, the administration frequency is reduced, the defect of existing marketed dosage forms is overcome; the preparation method has the advantages of good reproducibility, good repeatability, and easiness for being strongly implemented and popularized on the industry.
Owner:XUZHOU MEDICAL UNIV
Formula of degreasing liquid in IgY preparation process and preparation method of formula
InactiveCN105541999AEasy extractionReasonable designEgg immunoglobulinsPeptide preparation methodsPluronic F68Yolk
The invention discloses a formula of degreasing liquid in an IgY preparation process. The formula is prepared from the following components according to the ratios: 2 to 5 parts of Pluronic F68, 5 to 9 parts of PEG6000 dry powder, 1 to 3 parts of CaCl2, 0.3 to 1.5 parts of CH3COONa and the balance of double distilled water. The formula disclosed by the invention is reasonable in design; an antibody can be rapidly and simply extracted aiming at immune eggs; almost lipids and lipoprotein in yolks can be removed; particularly, identification shows that the lipid residue of a purified IgY antibody is less than 8 percent; after the purified IgY antibody is identified by SDS-PAGE, the purity has no difference from that of a common water extraction method and the like.
Owner:NORTHWEST A & F UNIV
Serum-free culture medium of placental mesenchymal stem cells
ActiveCN111593019APromote rapid proliferationImprove proliferative abilityCulture processSkeletal/connective tissue cellsTransferrinBiophysics
The invention relates to the technical field of stem cells, in particular to a serum-free culture medium of placental mesenchymal stem cells. The serum-free culture medium of the placental mesenchymalstem cells comprises a DMEM low-sugar culture medium and a supplement, wherein the supplement comprises the following components: human serum albumin with a concentration of 1-5 mg / mL, transferrin with a concentration of 5-10 [mu]g / mL, an epidermal growth factor with a concentration of 5-10 ng / mL, a transforming growth factor with a concentration of 10-30 ng / mL, a platelet-derived growth factor with a concentration of 10-25 ng / mL, glutathione with a concentration of 1-5 [mu]g / mL, Pluronic F68 with a concentration of 10-20 [mu]g / mL and lignans with a concentration of 5-10 [mu]g / mL. The produced serum-free culture medium can significantly improve the proliferation capability of the placental mesenchymal stem cells, a survival rate of the placental mesenchymal stem cells is high, the culturecost is low, the culture time of the cells is shortened, the application range is wide, and the serum-free culture medium of the placental mesenchymal stem cells can be used for industrial production.
Owner:广州同康生物科技有限公司
A drug-loaded fat emulsion co-loaded with oxaliplatin and irinotecan and its preparation method
ActiveCN106619509BImprove physical stabilityGood biocompatibilityOrganic active ingredientsOrganic non-active ingredientsPluronic F68Glycerol
The invention discloses an oxaliplatin-and-irinotecan jointly loading lipid emulsion prepared based on the drug lipid composite technology. The oxaliplatin-and-irinotecan jointly loading lipid emulsion is prepared from oxaliplatin with the concentration of 0.5 mg / ml-2 mg / ml, irinotecan with the concentration of 0.5 mg / ml-5 mg / ml, 5%-20% of injection oil, 0.5%-2.5% of phospholipid, 0.1%-2% of pluronic F68, 0.01%-0.5% of a stabilizing agent, 0.1%-2.5% of glycerol and water. By means of the jointly-loading lipid emulsion prepared with the drug lipid composite technology, synchronous releasing of two drugs in the human body can be coordinated, it is guaranteed that the two drugs are kept in the optimized dosage ratio when reaching the tumor site, and therefore the best collaborative effect is achieved.
Owner:SHANDONG UNIV
An anti-tumor drug-resistant nano-delivery system encapsulating two surfactants and preparation method thereof
ActiveCN104382866BGood anti-drug resistanceGood biocompatibilityOrganic active ingredientsPowder deliverySolubilityFreeze-drying
The invention relates to the technical field of medicines, provides a nano-scale delivery system loading two surfactants and having anti-tumor-drug-resistance, and further provides a preparation method of the nano-scale delivery system and application of the nano-scale delivery system in preparation of anti-tumor medicines. Two surfactants loaded in the drug-loaded nano-particles are Pluronic L61 and Pluronic F68, wherein Pluronic L61 has excellent anti-drug-resistance and excellent biocompatibility, has the activity independent of types of chemotherapeutic drugs and tumors and can be widely applied to chemosensitization of multiple tumors and Pluronic F68 has excellent water solubility and safety, is capable of remarkably improving stability of nano-particles in water and guaranteeing in-vivo long circulation of the nano-particles to reach tumor tissues and further has an effect of freeze-drying protection and no extra freeze-drying protective additive needs to be added.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Poly(vinyl benzoate) nanoparticles for molecular delivery
The present invention comprises poly(vinyl benzoate) nanoparticle suspensions as molecular carriers. These nanoparticles can be formed by nanoprecipitation of poly(vinyl benzoate) in water using Pluronic F68 as surfactant, to create spherical nanostructures measuring about 200-250 nm in diameter which are stable in phosphate buffer and blood serum, and only slowly degrade in the presence of esterases. Kinetics experiments in phosphate buffer indicate that 78% of the coumarin-6 was encapsulated within the polymer matrix of the nanoparticle, and the residual 22% of coumarin-6 was surface-bound and quickly released. The nanoparticles are non-toxic in vitro towards human epithelial cells (IC50>1000 μg / mL) and primary bovine primary aortic endothelial cells (IC50>500 μg / mL), and exert non-observable bactericidal activity against a selection of representative test microbes (MIC>250 μg / mL). Poly(vinyl benzoate) nanoparticles are suitable carriers for molecular delivery of lipophilic small molecules such as drugs pharmaceutical and imaging agents.
Owner:UNIV OF SOUTH FLORIDA
A kind of nimodipine oral long-acting suspension and preparation method thereof
ActiveCN108403629BEasy to takeGreat tasteOrganic active ingredientsSenses disorderOral suspensionsNimodipine
The invention discloses a nimodipine oral long-acting suspension and a preparation method thereof. -2 parts, 1-5 parts of pH buffer, 0.1-2 parts of preservative, 90-100 parts of solvent; the carrier material of nimodipine nanoparticles is the composition of polylactic acid-glycolic acid copolymer and pluronic F68, The mass percentage of Pluronic F68 in the carrier material is 5-40%. The nimodipine oral long-acting suspension of the present invention is convenient to take and has a good mouthfeel; the existence of some medicines in a free state can accelerate the absorption rate and have a quick effect; In granules, the first-pass effect can be reduced, the utilization rate of drugs is high, the drug loading capacity of nanoparticles is large, the encapsulation rate is high, the release is slow, and the action time is long, which reduces the frequency of administration and makes up for the shortcomings of existing commercially available dosage forms; preparation The method has good reproducibility and repeatability, and is easy to implement and popularize vigorously in the industry.
Owner:XUZHOU MEDICAL UNIV
Antibacterial peptide preservative, antibacterial peptide preparation containing the same, and preparation method for the antibacterial peptide preparation
ActiveCN102205128BNo reduction in biological activityImprove or maintain stabilityAntibacterial agentsPeptide/protein ingredientsArginineAntioxidant
The invention discloses an antibacterial peptide preservative, an antibacterial peptide preparation containing the same, and a preparation method for the antibacterial peptide preparation, and belongs to the field of pharmaceutical preparation. The antibacterial peptide preservative comprises a buffer, an amino acid, a stabilizing agent and an antioxidant. The antibacterial peptide preparation comprises the antibacterial peptide, a buffer, an amino acid, a stabilizing agent and an antioxidant. The preparation method for the antibacterial peptide preparation comprises: uniform mixing the antibacterial peptide, the buffer, the amino acid, the stabilizing agent, the antioxidant and water for injection, followed by adjusting pH value of the resulting mixture to 8.0-10.5, wherein the buffer is a Tris buffere, the amino acid is arginine, lysine or glutamic acid, the stabilizing agent is tween-80, Pluronic F68 or Pluronic F88, the antioxidant is methionine. According to the technical scheme provided by the embodiments of the present invention, the antibacterial peptide can be preserved for a long time without biological activity reducing, and preparation technology is simple so as to be suitable for an industrial production.
Owner:QINGDAO VLAND BIOTECH INC +1
Naringenin nano lipid carrier and its preparation method and application
ActiveCN111228220BEasy to prepareEasy to controlOrganic active ingredientsDigestive systemPolyoxyethylene castor oilGlycerol
The invention provides a naringenin nano lipid carrier as well as a preparation method and application thereof. The naringenin nano lipid carrier comprises naringenin and a nano lipid carrier. When the naringenin nano lipid carrier is prepared by using a rotational evaporation method, the nano lipid carrier comprises phospholipid, glycerol trilaurate, medium chain triglyceride and polyoxyethylatedcastor oil; and when the naringenin nano lipid carrier is prepared by using an emulsification evaporation-low temperature curing method, the nano lipid carrier comprises phospholipid, glyceryl monostearate, stearic acid, oleic acid and pluronic F68. The naringenin nano lipid carrier provided by the invention has high encapsulation efficiency and a large medicine carrying capacity, and the orallytaken bioavailability of naringenin can be improved. Compared with free naringenin and a naringenin nano lipid of a reference file 3, the naringenin nano lipid carrier prepared by using the emulsification evaporation-low temperature curing method has higher trans-enterocyte single-layer transfer efficiency and better small intestine absorption effects. Therefore, the carrier has a good inhibitionfunction on non-alcoholic fatty liver diseases with a small dosage.
Owner:PEKING UNIV
Liquid pharmaceutical formulations of FSH and LH together with a non-ionic surfactant
ActiveCN100488566CPeptide/protein ingredientsPharmaceutical non-active ingredientsFollicle-stimulating hormonePluronic F68
The present invention relates to the field of pharmaceutical preparations of follicle stimulating hormone (FSH), luteinizing hormone (LH) and mixtures of FSH and LH and methods for the production of these preparations. The present invention also provides liquid or lyophilized formulations of FSH, or LH, or FSH and LH, which contain a surfactant selected from the group consisting of Pluronic(R) F77, Pluronic F87, Pluronic F88, and Pluronic F68.
Owner:ARES TRADING SA
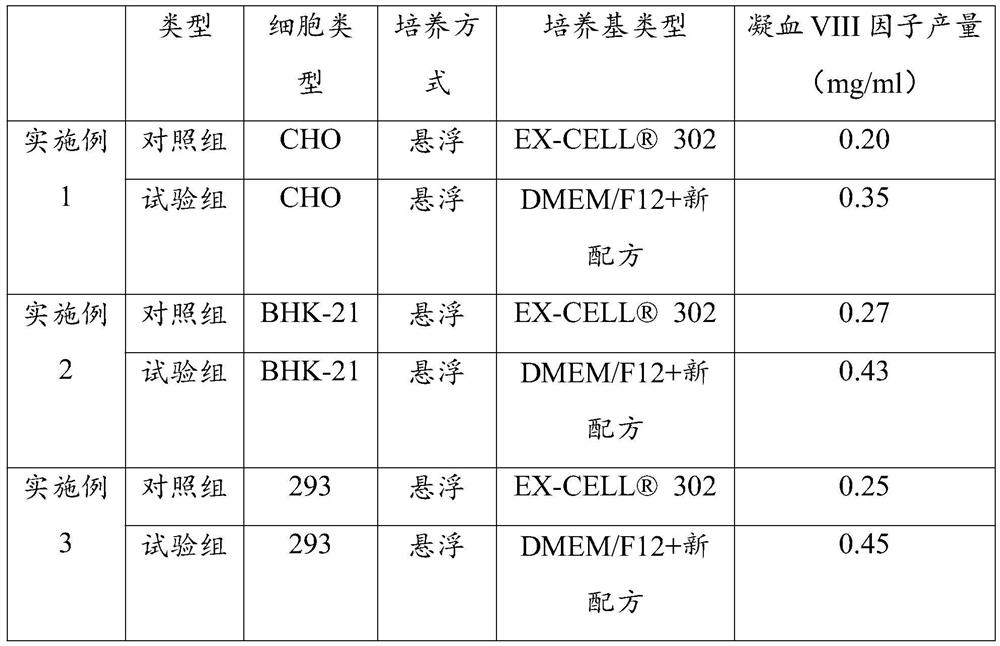
Culture medium additive for promoting protein expression and application thereof
The invention discloses a culture medium additive for promoting protein expression, and belongs to the technical field of cell culture. The culture medium additive comprises vitamin C, ethanolamine, L-asparagine, L-cysteine hydrochloride, L-cystine dihydrochloride, L-proline, L-tryptophan, soy peptone, N-acetylcysteine and pluronic F68. The culture medium additive for promoting protein expression is added into DMEM, DMEM / F12 and RPMI1640 basic culture media, so that the yield of cell expression protein can be increased, and a foundation is laid for improving protein antibody drugs.
Owner:内蒙古金源康生物工程股份有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com