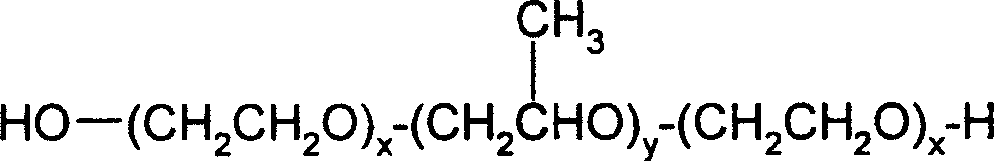
Liquid pharmaceutical formulations of FSH and LH together with a non-ionic surfactant
A surfactant, liquid drug technology, used in luteinizing hormone preparations, FSH and LH mixtures, and follicle-stimulating hormone pharmaceutical preparations, which can solve problems such as disappearance of biological efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
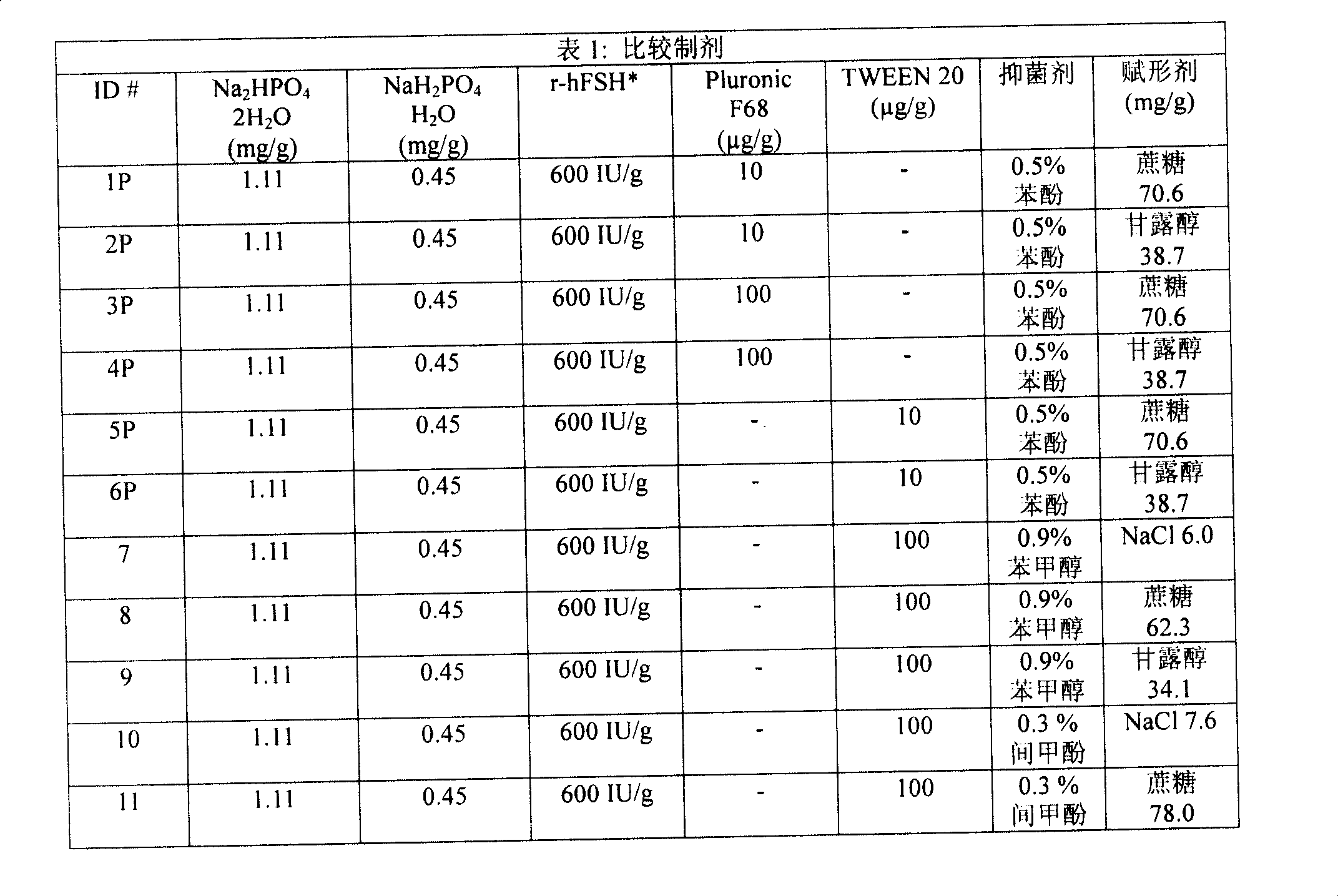
[0228] Comparative preparation
[0229] Material
[0230] name manufacturer r-hFSH Bulk Solution for Candidate Formulations Laboratoires Serono SA D-Mannitol
(DAB, Ph Eur, BP, FU, USP, FCC, E421) Merck sucrose
(DAB, Ph Eur, BP, NF) Merck NaCl (ACS, ISO) Merck Na 2 HPO 4 2H 2 o
(analytical pure) Merck NaH 2 PO 4 h 2 o
(analytical pure) Merck
[0231] name manufacturer Benzyl alcohol
(analytical pure) Merck
m-cresol
(for synthesis) Merck
TWEEN 20 (Polysorbate 20)
(for synthesis) Merck
Pluronic F68 (Poloxamer 188) Sigma L-methionine
(for biochemistry) Merck
Orthophosphoric acid 85%
(Ph Eur, BP, NF) Merck
1.5mL glass syringe
SFAM
(siliconed at Aguettant) Type A rubber West Company Crim cover Aguettant Millex-GV
Syringe Driven Filters - Durapore Millipore
Durapore filter membrane 0...
Embodiment 2
[0264] Liquid single-dose formulation of recombinant FSH for subcutaneous or intramuscular injection
[0265] Based on the results of Example 1, the following formulations were prepared.
[0266] Components 1 to 7 listed in Table 3 were prepared as equivalent standard solutions in WFI. Add aliquots of each solution to a mixing vessel to form a "mother solution." This stock solution was dispensed into vials containing 10.9 micrograms (150 IU) or 5.45 micrograms (75 IU) of FSH.
[0267] Because of the use of recombinant FSH, the biological activity and specificity are consistent, so that a large amount of filling FSH (fill-by-mass) can be achieved instead of filling through biological tests.
[0268]
[0269] Fill and seal vials under aseptic conditions. These preparations can be stored at room temperature for up to 2 years.
Embodiment 3
[0271] Liquid multiple-dose formulation of recombinant FSH for subcutaneous or intramuscular injection
[0272] Based on the results of Example 1, the following multi-dose formulations were prepared.
[0273] Components 1 to 7 listed in Table 4 were prepared as equivalent standard solutions in WFI. Add aliquots of each solution to a mixing vessel to form a "mother solution." This stock solution was dispensed into vials containing 22.2 micrograms (305 IU), 33.3 micrograms (458 IU) and 66.7 micrograms (916 IU) of FSH. The resulting formulations delivered a total of 300, 450 and 900 IU FSH.
[0274] Fill and seal syringe barrels under aseptic conditions. These multi-dose formulations may be stored at about 2-8°C, preferably at about 4-5°C, up to a maximum of 2 years after first use. After first use, the syringe should be stored at about 2-8°C, preferably at about 4-5°C, for a period of 24 hours, 2 days, or up to 12 or 14 days.
[0275]
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com