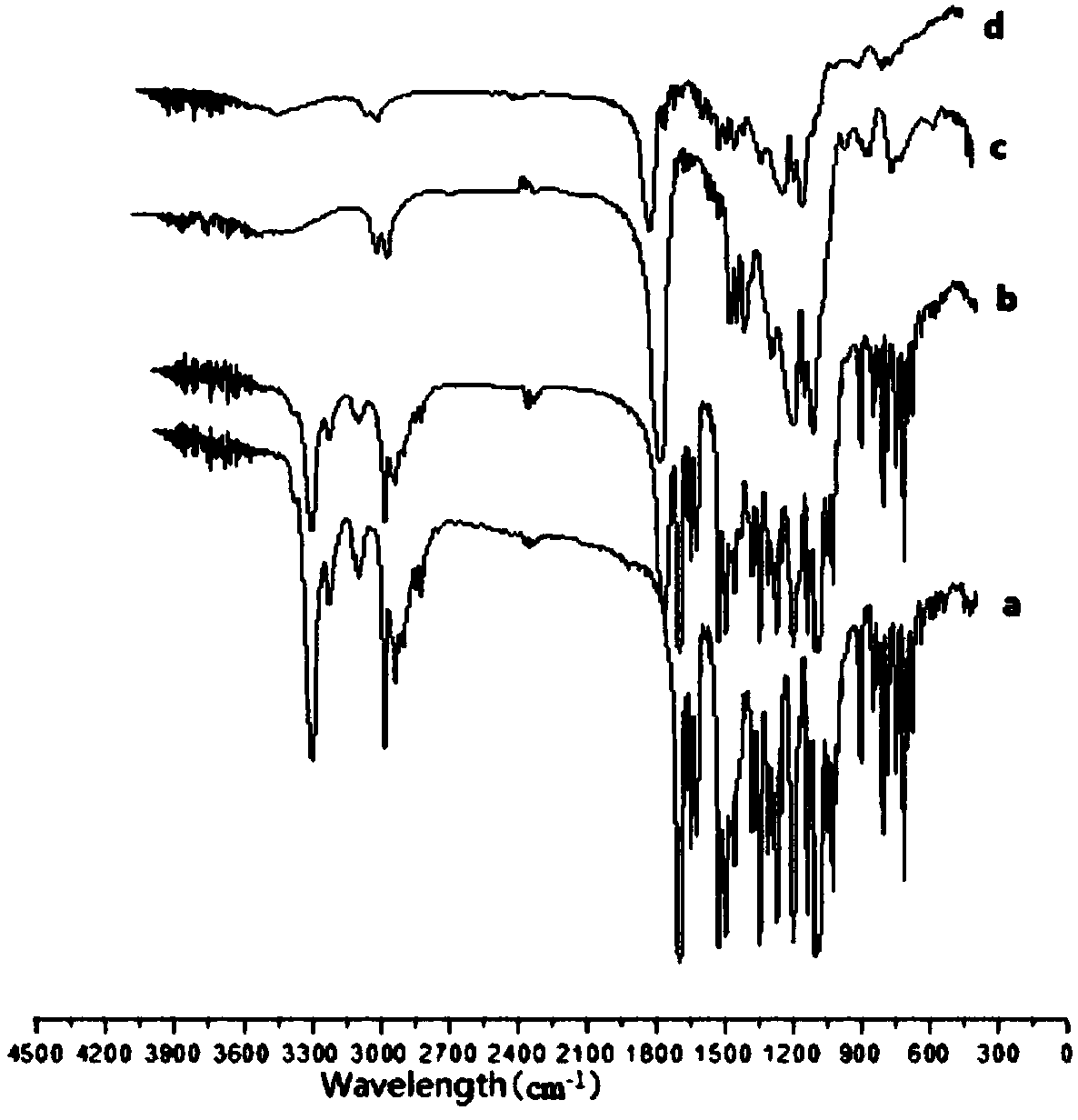
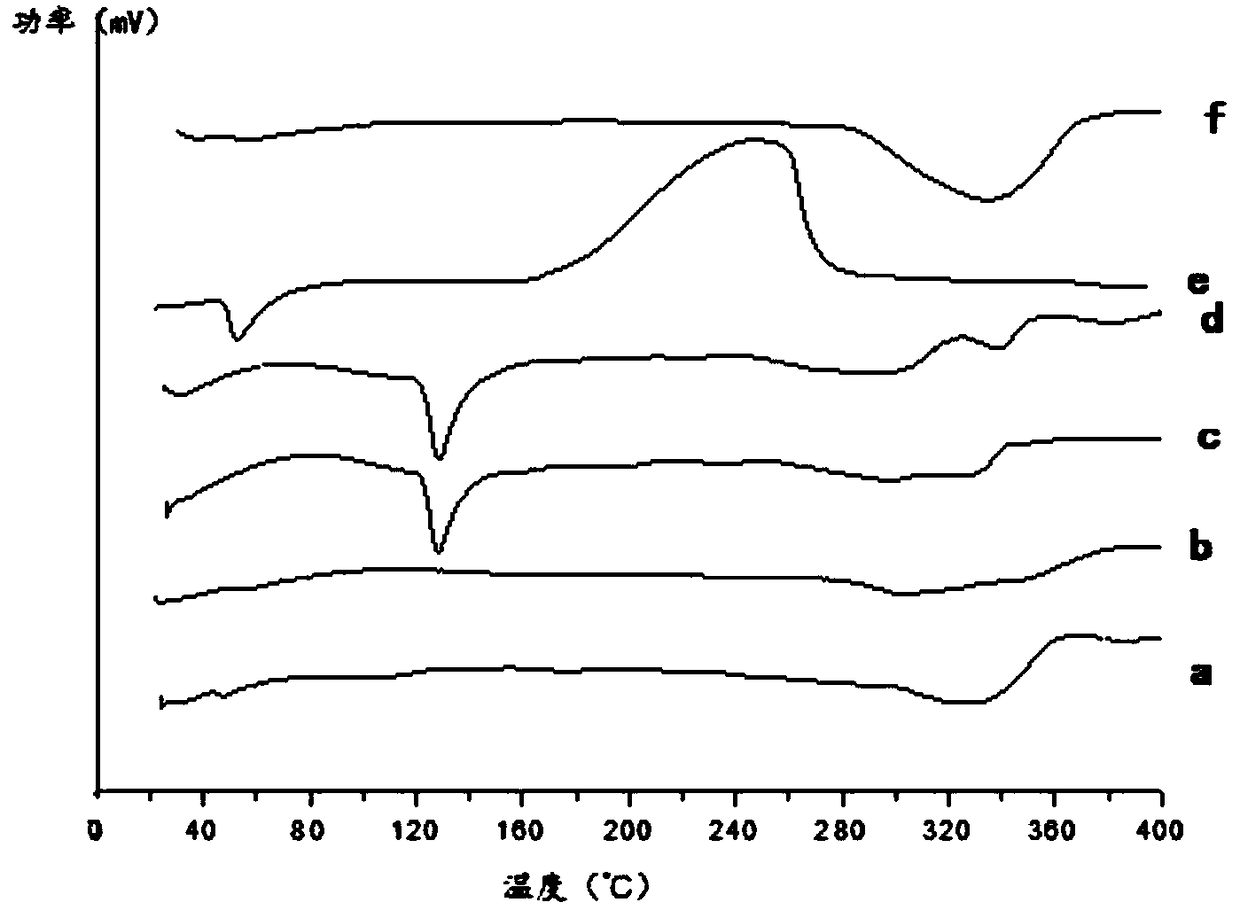
Nimodipine long-acting oral suspension liquid and preparation method thereof
A nimodipine and nimodipine nano-technology, applied in the field of medicine, can solve problems such as unclear effectiveness and safety, low bioavailability, and insoluble drugs in water, so as to improve drug encapsulation rate and drug utilization High rate, increase the effect of effective blood concentration time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Table 1 Prescription of nimodipine oral solution
[0043]
[0044]
[0045] Preparation Process:
[0046] (1) Get 0.75g nimodipine, 0.5g benzoic acid and disperse in 100ml ethanol;
[0047] (2) Add 150ml polyethylene glycol and 125ml glycerin to the above solution, and mechanically stir for 10min until dissolved;
[0048] (3) Take 60ml of purified water and add the prescription amount of sodium dihydrogen phosphate and disodium hydrogen phosphate and mechanically stir for 10 minutes until dissolved;
[0049] (4) Add the prescription amount of maltitol to the solution prepared in (3) and mechanically stir for 10 minutes until dissolved;
[0050](5) Add the solution prepared in (4) above to the solution prepared in (2), and stir mechanically for 10 minutes;
[0051] (6) Dilute the purified water to the prescribed amount, and filter with a 0.45 micron filter element.
Embodiment 2
[0053] Table 2 Prescription of nimodipine oral solution
[0054]
[0055]
[0056] Preparation Process:
[0057] (1) Disperse 0.75g of nimodipine in 100ml of ethanol.
[0058] (2) Add 125ml of polyethylene glycol, 150ml of glycerin, and 5ml of benzyl alcohol to the above solution, and stir mechanically for 10 minutes until dissolved.
[0059] (3) Take 60ml of purified water, add the prescribed amount of citric acid and sodium citrate, and mechanically stir for 10 minutes until dissolved.
[0060] (4) Add the prescribed amount of sucralose to the solution prepared in (3) and mechanically stir for 10 minutes until dissolved.
[0061] (5) Add the solution prepared in (4) above to the solution prepared in (2), and stir mechanically for 10 minutes.
[0062] (6) Dilute the purified water to the prescribed amount, filter with a 0.45 micron filter element, perform quality inspection, and pack in a brown glass bottle.
Embodiment 3
[0064] Example 3
[0065] After the sample was kept away from light and sealed for 3 months under accelerated stability (40°C, RH75%), the content of nimodipine was detected by liquid phase.
[0066]
[0067] We can find out by experiment, the stability of this invention prescription process nimodipine is high.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com