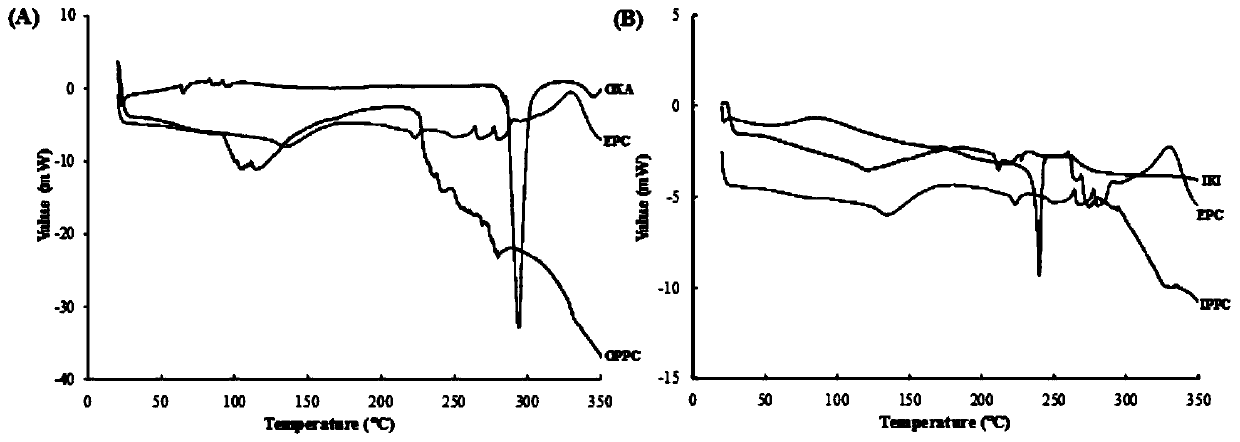
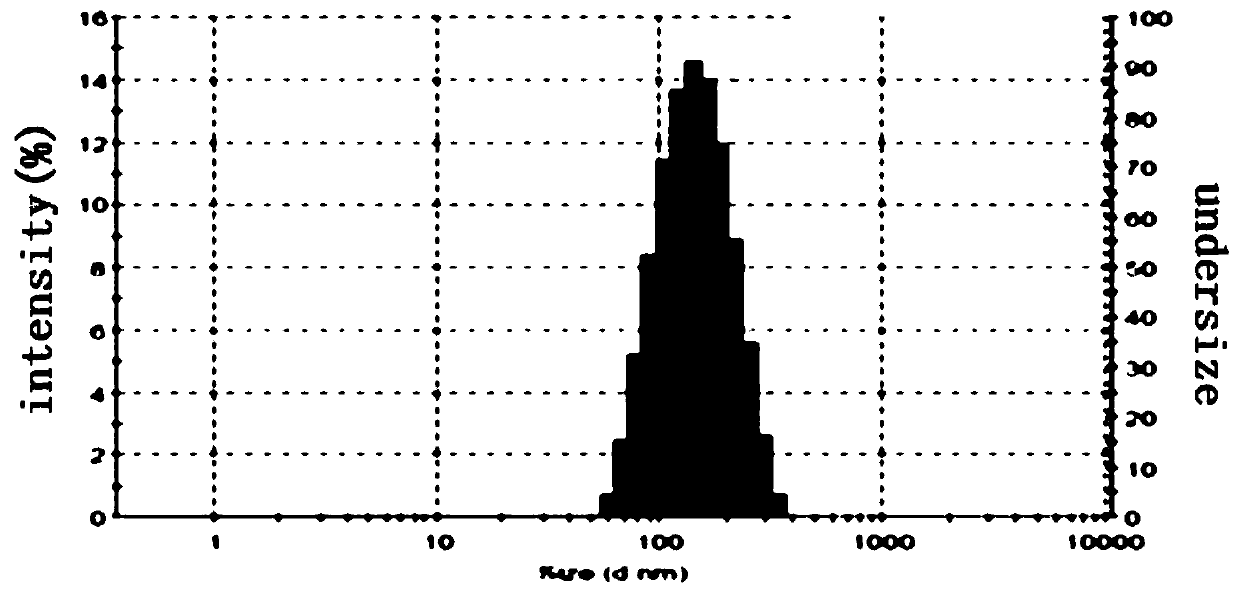
A drug-loaded fat emulsion co-loaded with oxaliplatin and irinotecan and its preparation method
A technology of irinotecan and oxaliplatin, which is applied in the field of oxaliplatin and irinotecan co-loaded drug-loaded fat emulsion and its preparation, which can solve the problems of co-loaded fat emulsion and improve the anti-tumor effect , outstanding synergistic effect, and good physical stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Example 1: Oxaliplatin and irinotecan were used to prepare drug lipid complexes respectively.
[0042] A fat emulsion co-carried with oxaliplatin and irinotecan, 20 mg of oxaliplatin and 300 mg of egg yolk phospholipids are dissolved in 20 ml of methanol / dichloromethane (volume ratio 9:1), 50 mg of irinotecan and 200 mg of egg yolk phospholipids are dissolved After reacting in 5ml of dichloromethane for a certain period of time, the two were vacuumized respectively to obtain oxaliplatin-lipid complex and irinotecan-lipid complex; the two drug-lipid complexes were redissolved in 4ml of dichloromethane , add MCT 2g, oleic acid 50mg, obtain oil phase after vacuumizing. 200 mg of Pluronic F68 and 800 mg of glycerin were dissolved in water to obtain an aqueous phase. After the oil phase and the water phase were preheated at 60°C respectively, they were mixed at a shear rate of 6000r / min to obtain colostrum, and water was added to make the final volume 40ml; followed by 15 c...
Embodiment 2
[0044] Example 2: Oxaliplatin and irinotecan were used to simultaneously prepare drug lipid complexes.
[0045] A kind of oxaliplatin and irinotecan co-carrying fat emulsion, take oxaliplatin 20mg, irinotecan 50mg and soybean lecithin 500mg and dissolve in methanol / methylene chloride 20ml (volume ratio 9:1), after reacting for a certain period of time , vacuumize to obtain oxaliplatin / irinotecan-lipid complex; redissolve the drug-lipid complex with 4ml of dichloromethane, add MCT 4g, oleic acid 50mg, and obtain an oil phase after vacuuming. 200 mg of Pluronic F68 and 900 mg of glycerin were dissolved in water to obtain an aqueous phase. After the oil phase and the water phase were preheated at 60°C respectively, they were mixed at a shear rate of 8000r / min to obtain colostrum, and water was added to make the final volume 40ml; followed by 15 cycles of high-pressure emulsification at 10Mpa to obtain co-loaded fat milk.
[0046] The concentrations of oxaliplatin and irinotecan...
Embodiment 3
[0047] Example 3: Oxaliplatin preparation of drug-lipoplexes and irinotecan were directly loaded.
[0048] A fat emulsion co-loaded with oxaliplatin and irinotecan. Take 20 mg of oxaliplatin and 300 mg of hydrogenated soybean lecithin and dissolve them in 20 ml of methanol / chloroform (volume ratio 9:1). After reacting for a certain period of time, vacuumize to obtain oxaliplatin Liplatin-lipid complex. The drug-lipid complex was redissolved with 4ml of chloroform, and 50mg of irinotecan, 2g of MCT, and 50mg of cholic acid were added, and an oily phase was obtained after vacuuming. 300 mg of Pluronic F68 and 800 mg of glycerin were dissolved in water to obtain an aqueous phase. After the oil phase and the water phase were preheated at 50°C, they were mixed at a shear rate of 10,000r / min to obtain colostrum, and water was added to make the final volume 40ml; then the high-pressure emulsion was circulated 10 times at 10Mpa to obtain co-loaded fat milk.
[0049] The concentrati...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com