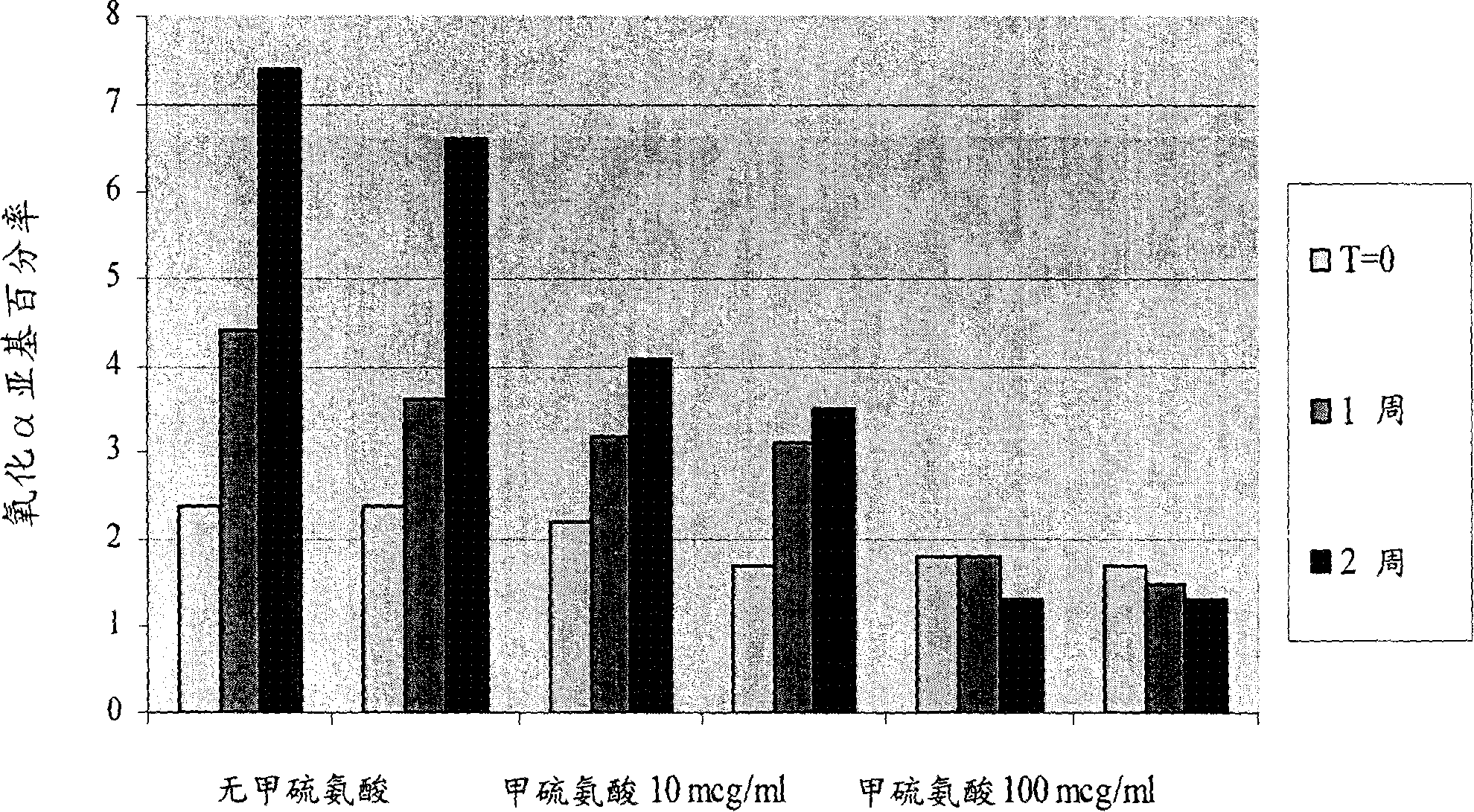
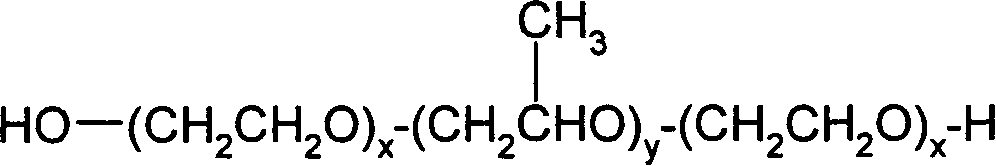
Liquid pharmaceutical formulations of fsh and lh together with a non-ionic surfactant
A surfactant and liquid drug technology, applied in the field of luteinizing hormone preparations, FSH and LH mixtures, and follicle stimulating hormone pharmaceutical preparations, can solve the problems of biological efficacy disappearance, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0226] Comparative preparation
[0227] name
manufacturer
r-hFSH Bulk Solution for Candidate Formulations
Laboratoires Serono SA
D-Mannitol
(DAB, Ph Eur, BP, FU, USP, FCC, E421)
Merck
(DAB, PhEur, BP, NF)
Merck
NaCl (ACS, ISO)
Merck
Na 2 HPO 4 2H 2 o
(analytical pure)
Merck
NaH 2 PO 4 h 2 o
(analytical pure)
Merck
name
manufacturer
(analytical pure)
Merck
m-cresol
(for synthesis)
Merck
TWEEN 20 (Polysorbate 20)
(for synthesis)
Merck
Pluronic F68 (Poloxamer 188)
Sigma
(for biochemistry)
Merck
Orthophosphoric acid 85%
(Ph Eur, BP, NF)
Merck
1.5mL glass syringe
SFAM
(siliconed at Aguettant)
Type A rubber
West Company
Crim cover
Aguettant
Millex-GV
Syrin...
Embodiment 2
[0253] Liquid single-dose formulation of recombinant FSH for subcutaneous or intramuscular injection
[0254] Based on the results of Example 1, the following formulations were prepared.
[0255] Components 1 to 7 listed in Table 3 were prepared as equivalent standard solutions in WFI. Add aliquots of each solution to a mixing vessel to form a "mother solution." This stock solution was dispensed into vials containing 10.9 micrograms (150 IU) or 5.45 micrograms (75 IU) of FSH.
[0256] Table 3. Components of FSH single-dose liquid formulations
[0257] Fill and seal vials under aseptic conditions. These preparations can be stored at room temperature for up to 2 years.
Embodiment 3
[0259] Liquid multiple-dose formulation of recombinant FSH for subcutaneous or intramuscular injection
[0260] Based on the results of Example 1, the following multi-dose formulations were prepared.
[0261] Components 1 to 7 listed in Table 4 were prepared as equivalent standard solutions in WFI. Add aliquots of each solution to a mixing vessel to form a "mother solution." This stock solution was dispensed into vials containing 22.2 micrograms (305 IU), 33.3 micrograms (458 IU) and 66.7 micrograms (916 IU) of FSH. The resulting formulations delivered a total of 300, 450 and 900 IU FSH.
[0262] Table 4. Components of FSH Multiple-Dose Liquid Formulations
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com