Naringenin nano lipid carrier and its preparation method and application
A nano-lipid carrier, naringenin technology, applied in the field of medicine, can solve the problems of low drug loading, drug leakage, etc., achieve the effect of simple preparation method, easy control and operation, and improved dissolution and absorption characteristics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] Embodiment 1, the preparation of naringenin nano lipid carrier (method 1)
[0066] Accurately weigh 0.021g of naringenin, 0.060g of soybean lecithin, 0.020g of medium-chain triglycerides, and 0.004g of glyceryl trilaurate, add 3.3mL of chloroform and 1.7mL of methanol, fully dissolve, and rotate under reduced pressure in a water bath at 37°C The solvent was removed by evaporation until a uniform lipid film formed on the sides of the eggplant-shaped flask. Add 3 mL of 0.01M phosphate buffer (pH=7.4) containing 230 mg of polyoxyethylene castor oil, and sonicate for 15 min (80% of full amplitude) in an ice bath to obtain the naringenin nano-lipid carrier 1 (NGN- NLC1).
Embodiment 2
[0067] Embodiment 2, the preparation of naringenin nano lipid carrier (method 2)
[0068] Accurately weigh 0.025g naringenin, 0.040g soybean lecithin, 0.020g stearic acid, 0.020g glyceryl monostearate, 0.020g oleic acid, add 2mL ethanol, fully dissolve, add 3% Pluronic F68 In 20mL of 0.01M phosphate buffer (pH=7.4), form an emulsion at 75°C until the concentration of naringenin is 8.3mg·mL -1 . The naringenin nano-lipid carrier 2 (NGN-NLC2) of the present invention was obtained by depositing and solidifying in an ice bath for 1 h.
Embodiment 3
[0069] Embodiment 3, the application of naringenin nano lipid carrier
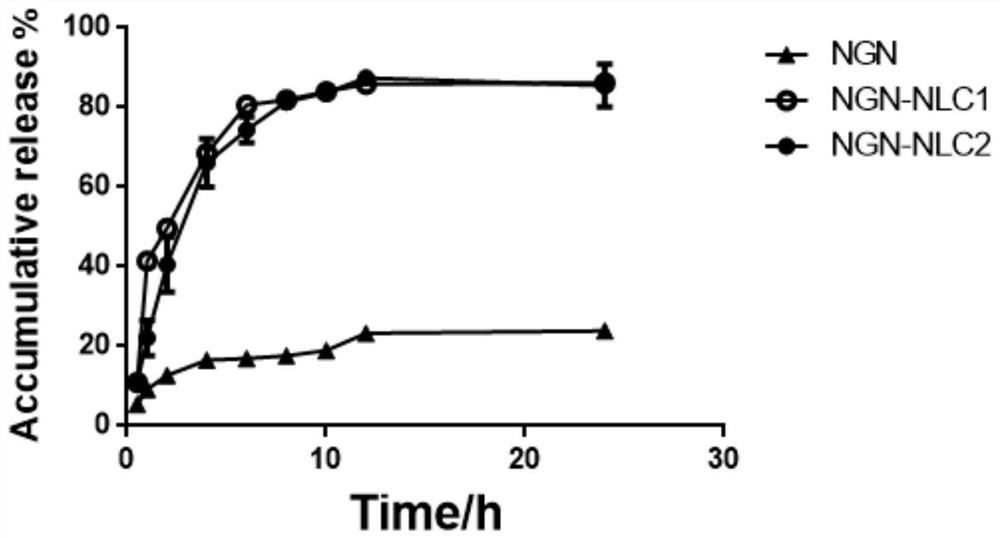
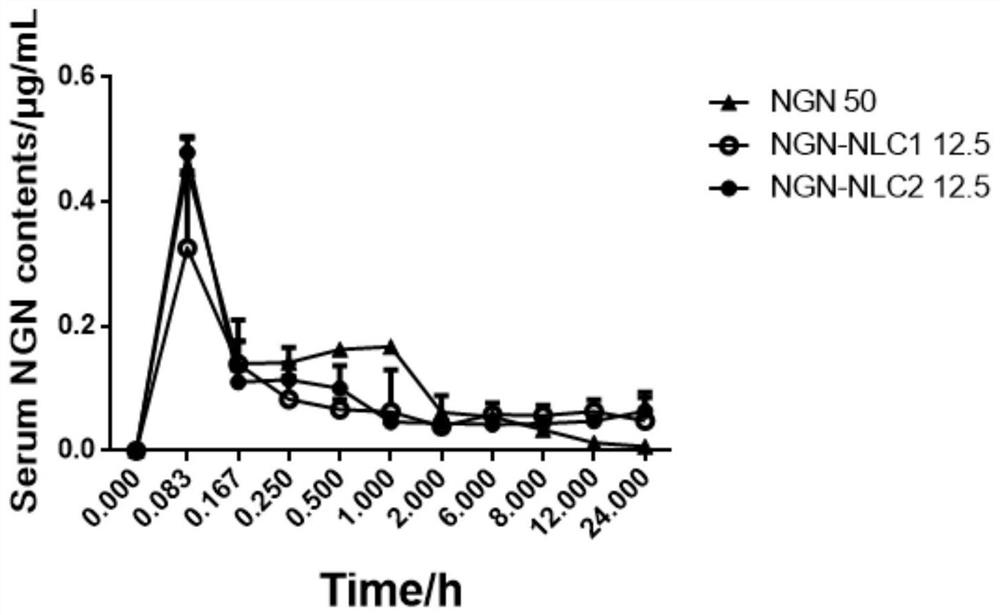
[0070] 1. Quality evaluation of naringenin nano-lipid carrier
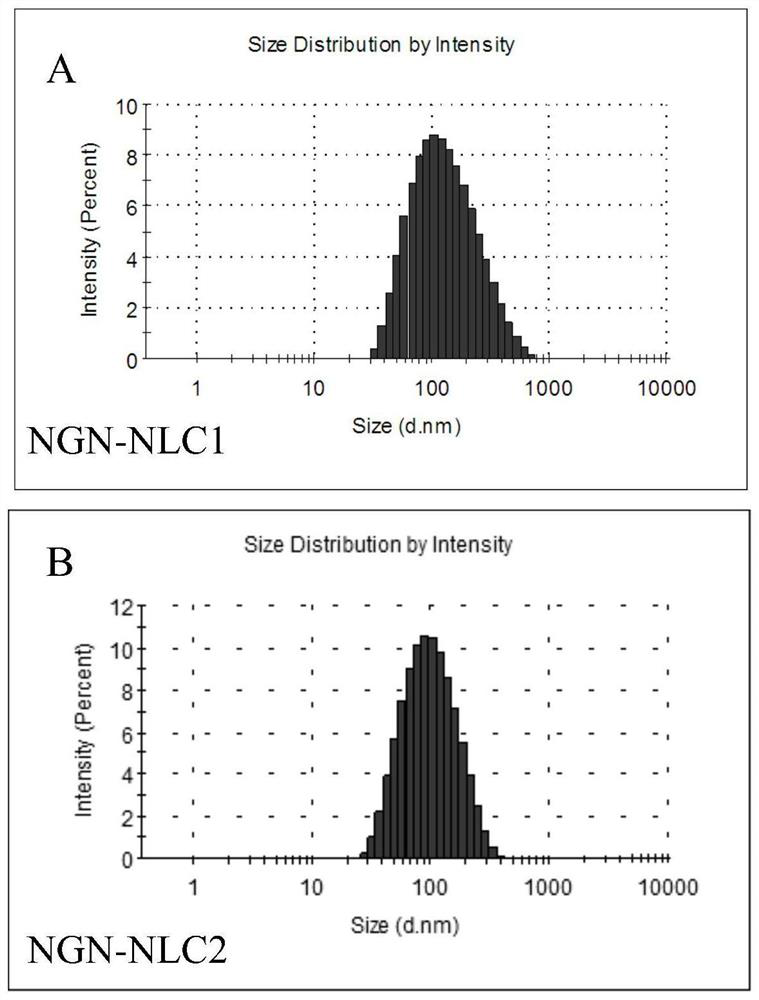
[0071] Particle size measurement: get an appropriate amount of NGN-NLC1 in Example 1 of the present invention, adopt a Malvern Zeta potentiometer to measure its particle size, and record the particle size as (120.8 ± 1.9) nm; get an appropriate amount of NGN-NLC2 in Example 2 of the present invention, and use a Malvern Zeta potentiometer to measure the particle size. The Zeta potential meter measures its particle size, and the measured particle size is (147.5±7.4) nm, and its particle size distribution diagram is as follows figure 1 shown.
[0072] Determination of Encapsulation Efficiency EE (%):
[0073] (1) HPLC method establishes naringenin standard curve:
[0074] Chromatographic conditions: Measuring instrument: LC-15 high performance liquid chromatography (Shimadzu Corporation, Japan) (with UV detector); Chromatographic column: C18 (...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| encapsulation rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com