Propofol microemulsion composition
A composition and technology of propofol, applied in the field of microemulsion preparation of propofol, can solve the problems of heat-labile, high hemolysis, high storage temperature requirements, etc., achieve good stability and safety, improve anti-inflammatory Activity, the effect of reducing adverse reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
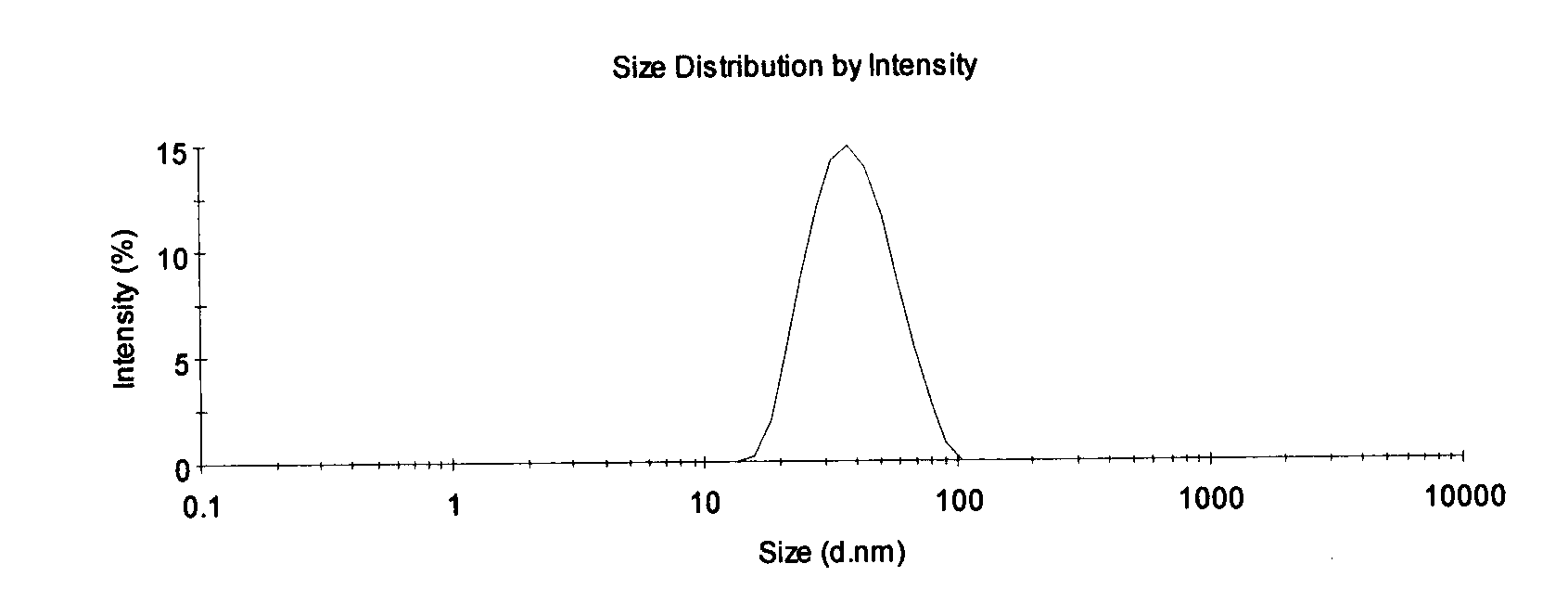
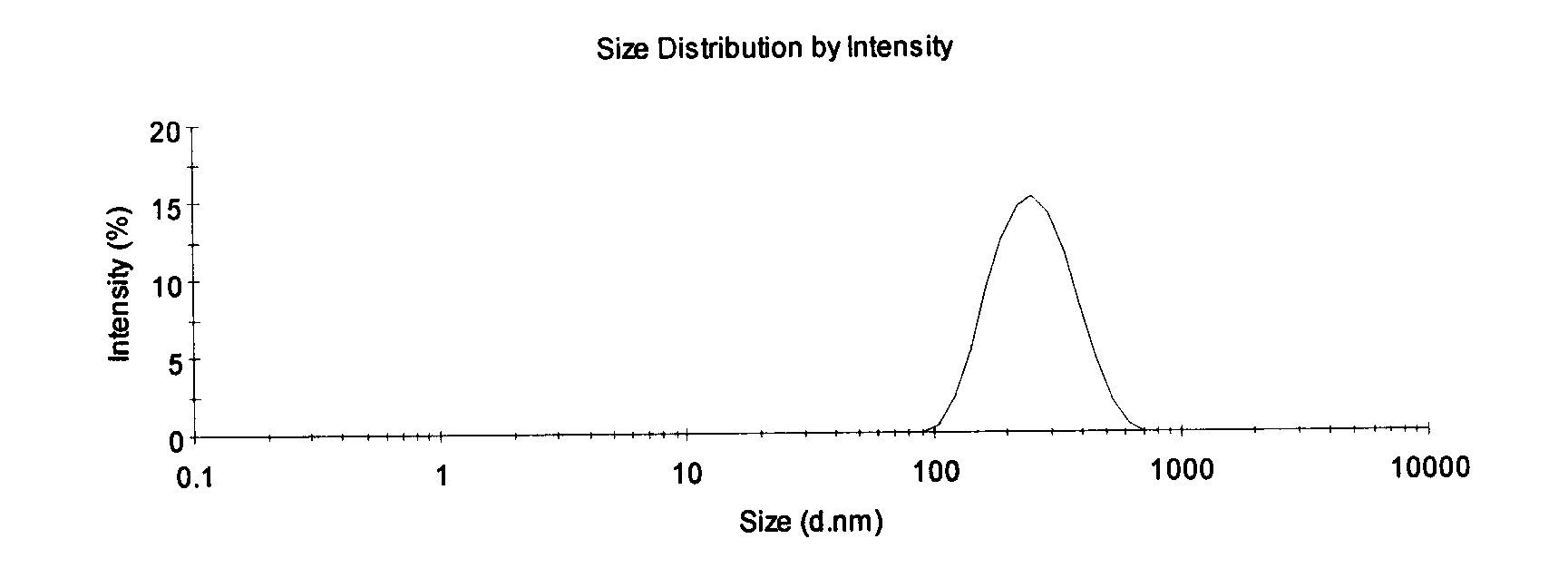
[0007] The invention relates to a propofol microemulsion composition. The microemulsion composition comprises main drug propofol and one or more auxiliary materials.
[0008] The adjuvant described in the present invention refers to the general term of all other substances except propofol, also referred to as additives or excipients or substrates, etc., which may be chemically inert.
[0009] In the present invention, the auxiliary material plays the role of emulsifier and / or co-emulsifier and / or solvent and / or bacteriostat.
[0010] The composition does not contain other polyoxyethylene surfactants, phospholipids and their derivatives, glycerides of medium, short, and long-chain fatty acids, cationic surfactants, etc. except the Pluronic series. Although these auxiliary materials can improve Propofol has problems such as low solubility in water and poor physical stability of the preparation, but it will destroy the composition of the microemulsion, leading to a series of hidd...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| polydispersity index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com