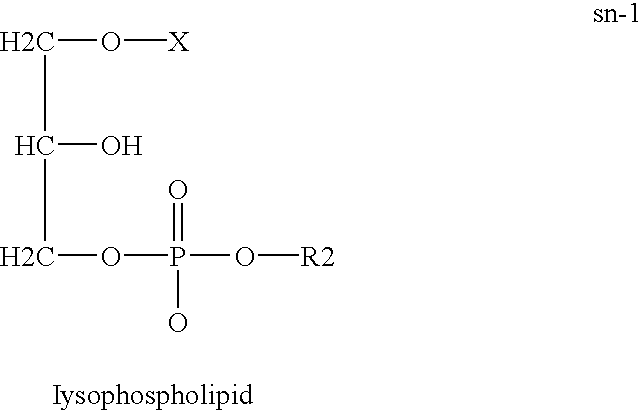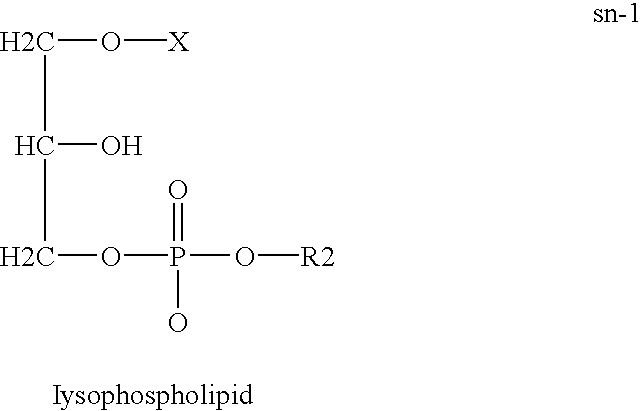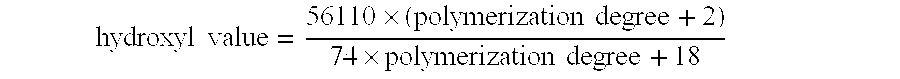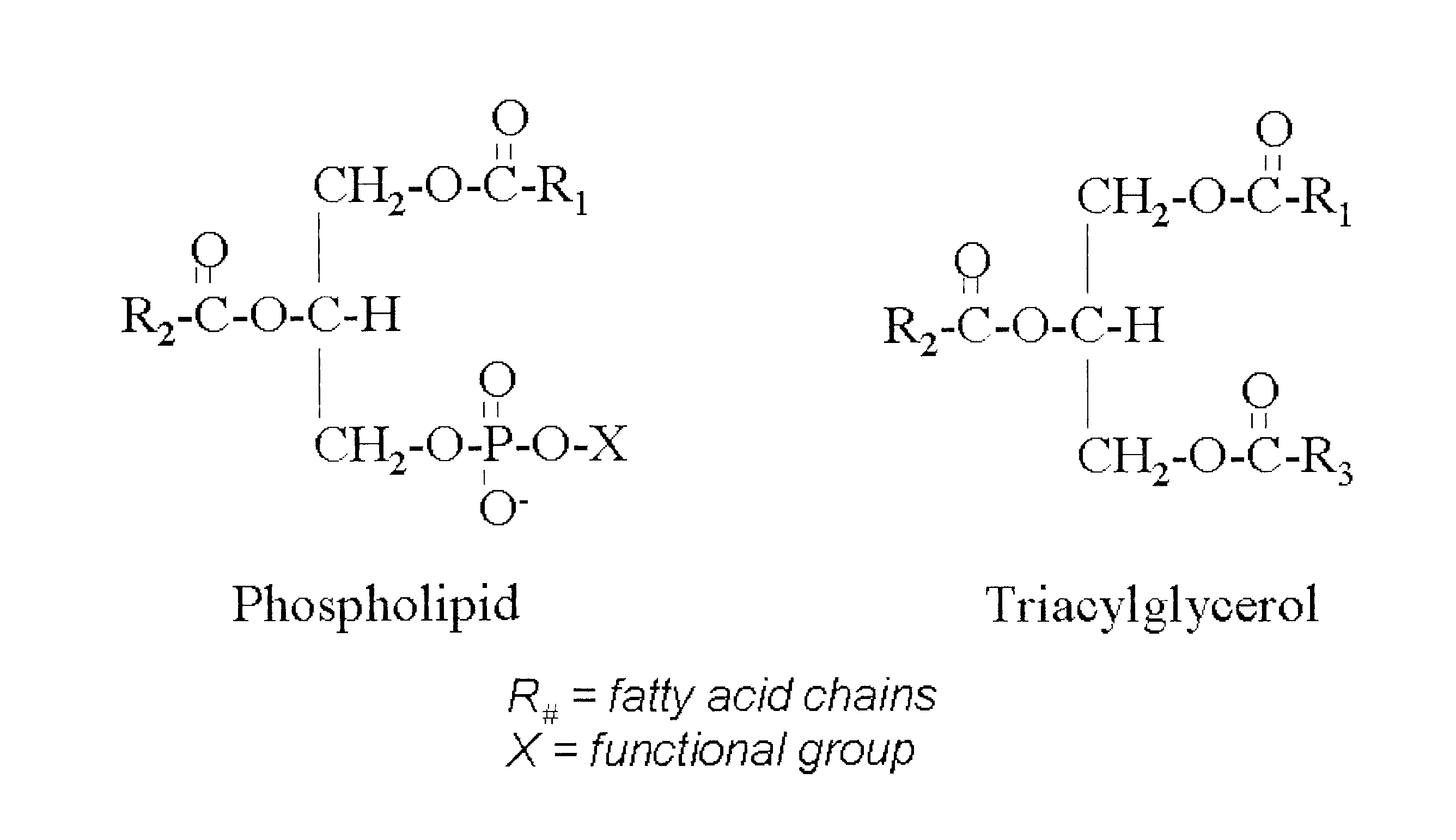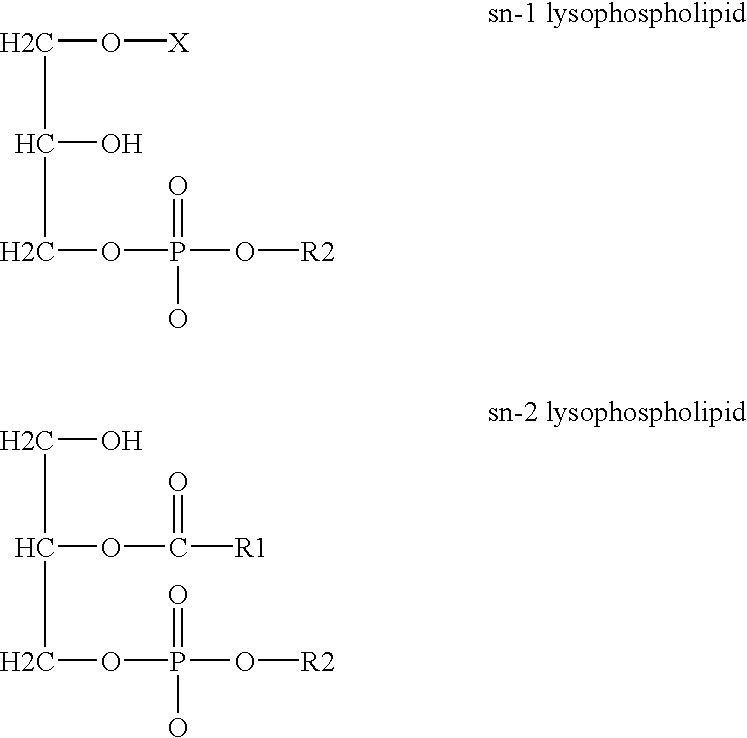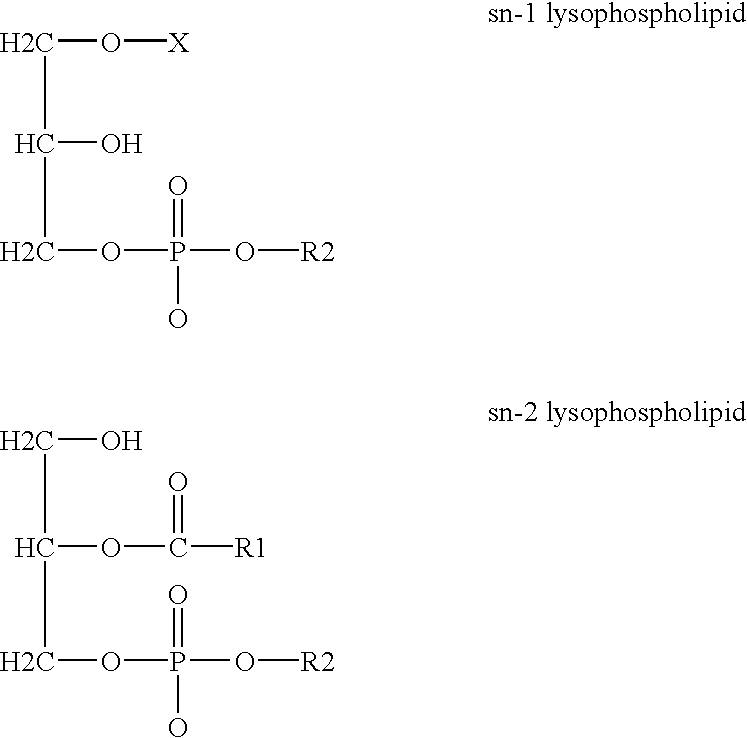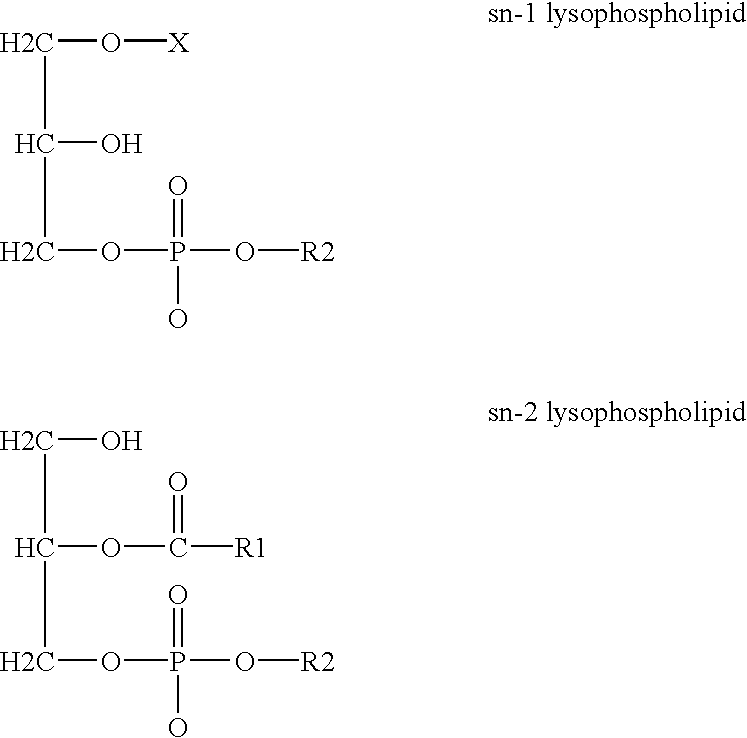Patents
Literature
95 results about "Lysophospholipids" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
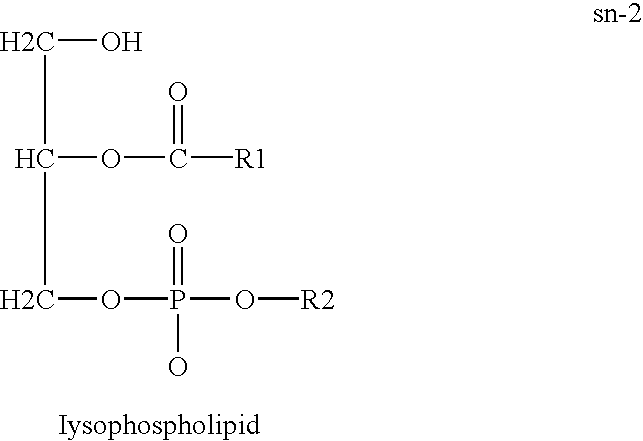
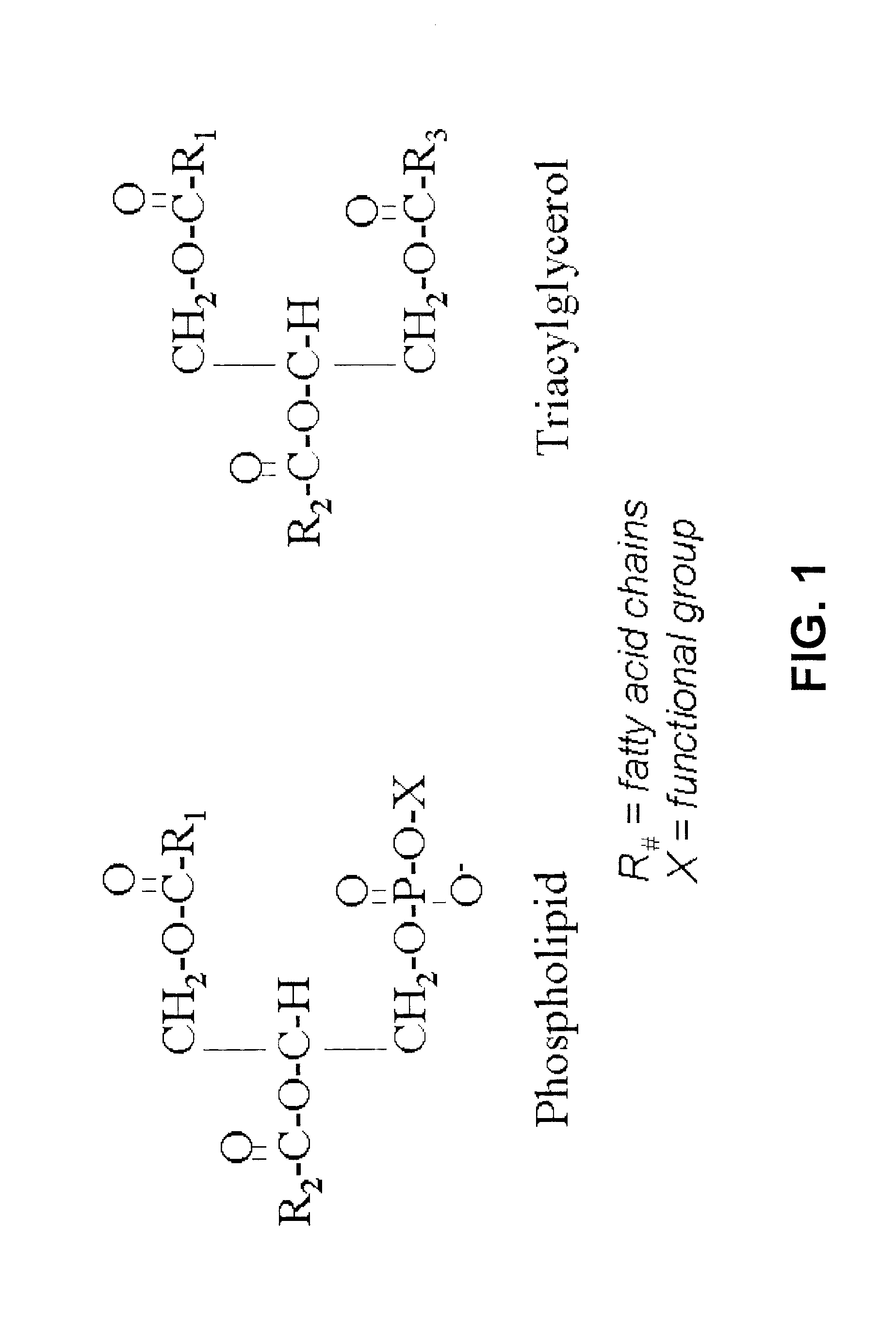
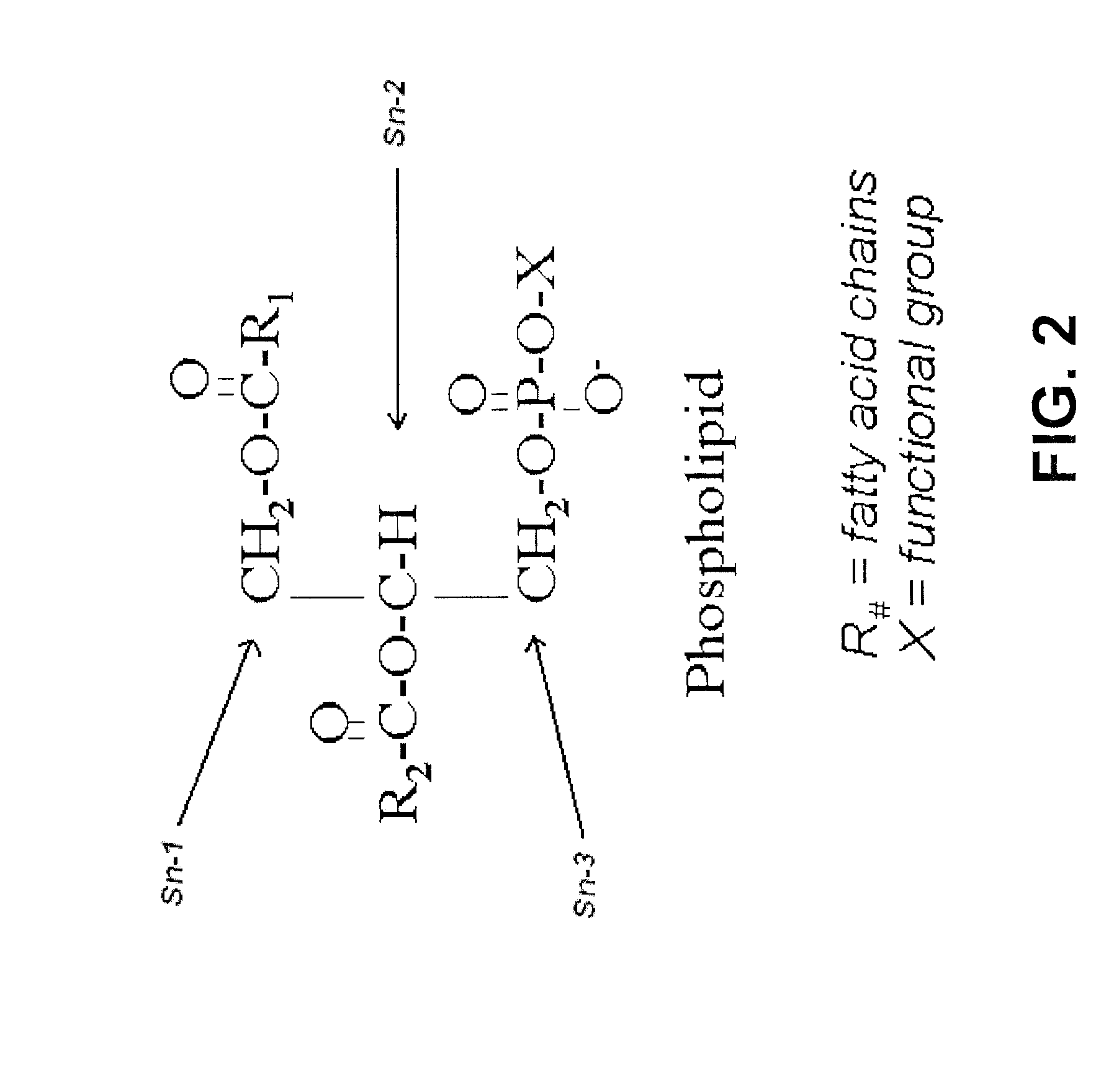
Derivatives of PHOSPHATIDIC ACIDS that lack one of its fatty acyl chains due to its hydrolytic removal.
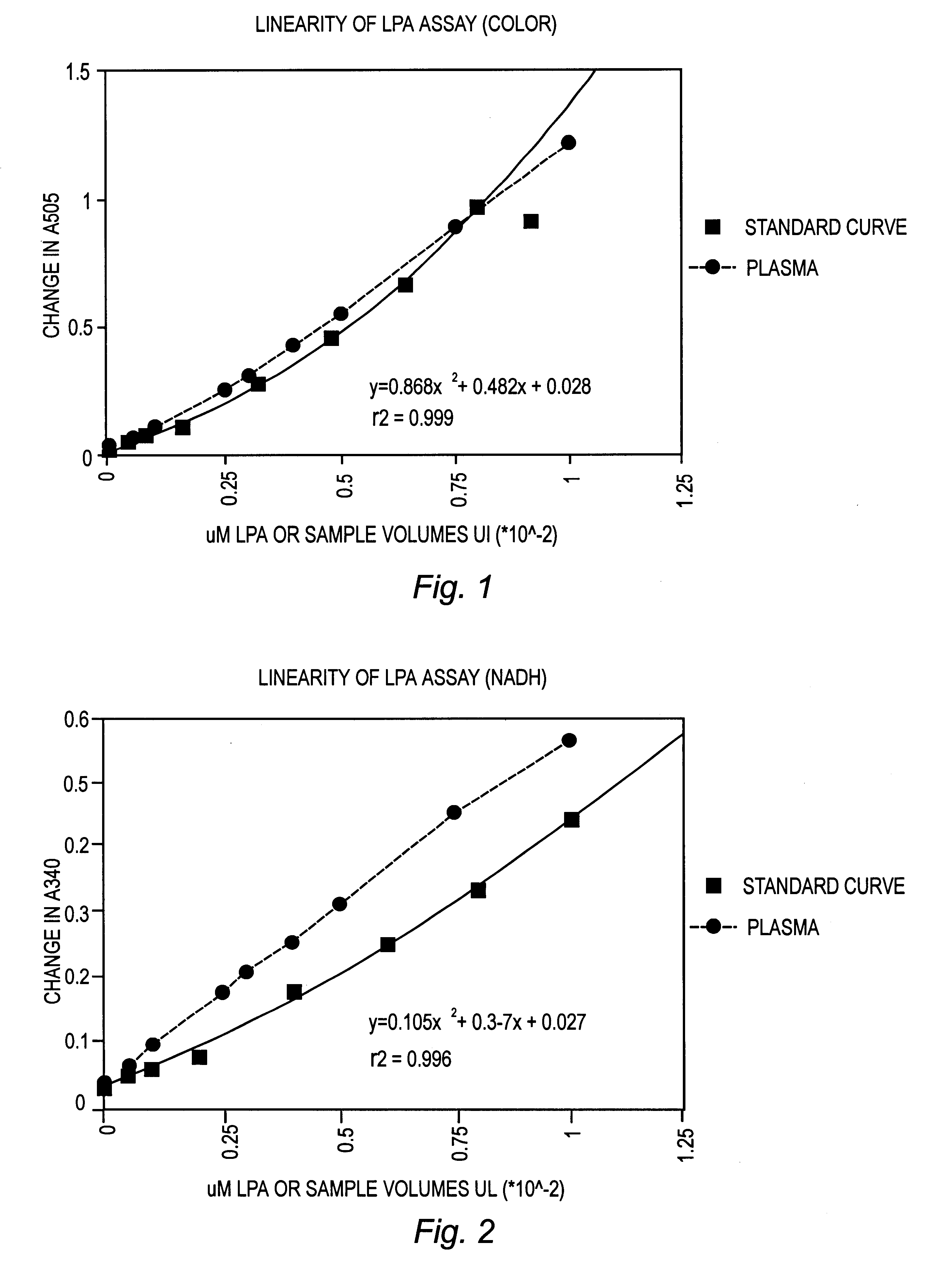
Enzyme method for detecting lysophospholipids and phospholipids and for detecting and correlating conditions associated with altered levels of lysophospholipids
InactiveUS6248553B1Enhanced signalMicrobiological testing/measurementBiological material analysisBody fluidBiology
The present invention is an enzymatic method and diagnostic kits for detecting and quantifying the presence of one or more lysophospholids in a sample of bodily fluid taken from a test subject. The method uses enzymes in a two step assay and may be used to detect disease conditions associated with altered levels of lysophospholipids and to correlate such conditions with altered levels of lysophospholipids.
Owner:APOLLO ENDOSURGERY INC
Invasomes for therapy of disorders, their preparation and use
The invention relates to invasomes comprising a lipid mixture comprising one or more lipids, preferably neutral lipids, one or more lysophosphatides and at least one pharmacological agent, preferably an immunomodulator, to the preparation thereof and to the use thereof for the therapy of disorders, preferably of disorders which can be treated by modulation of the immune system.
Owner:VECTRON THERAPEUTICS
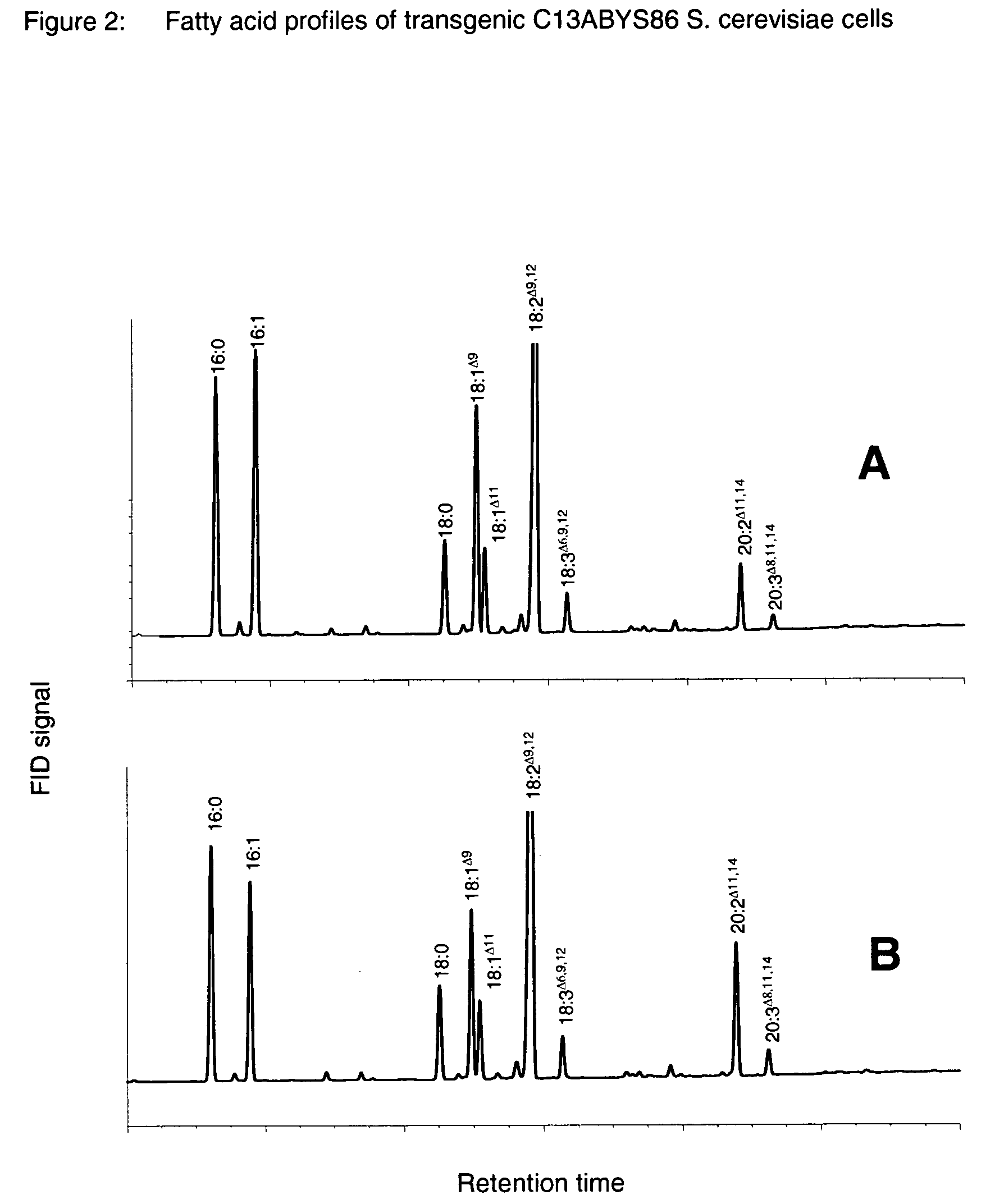
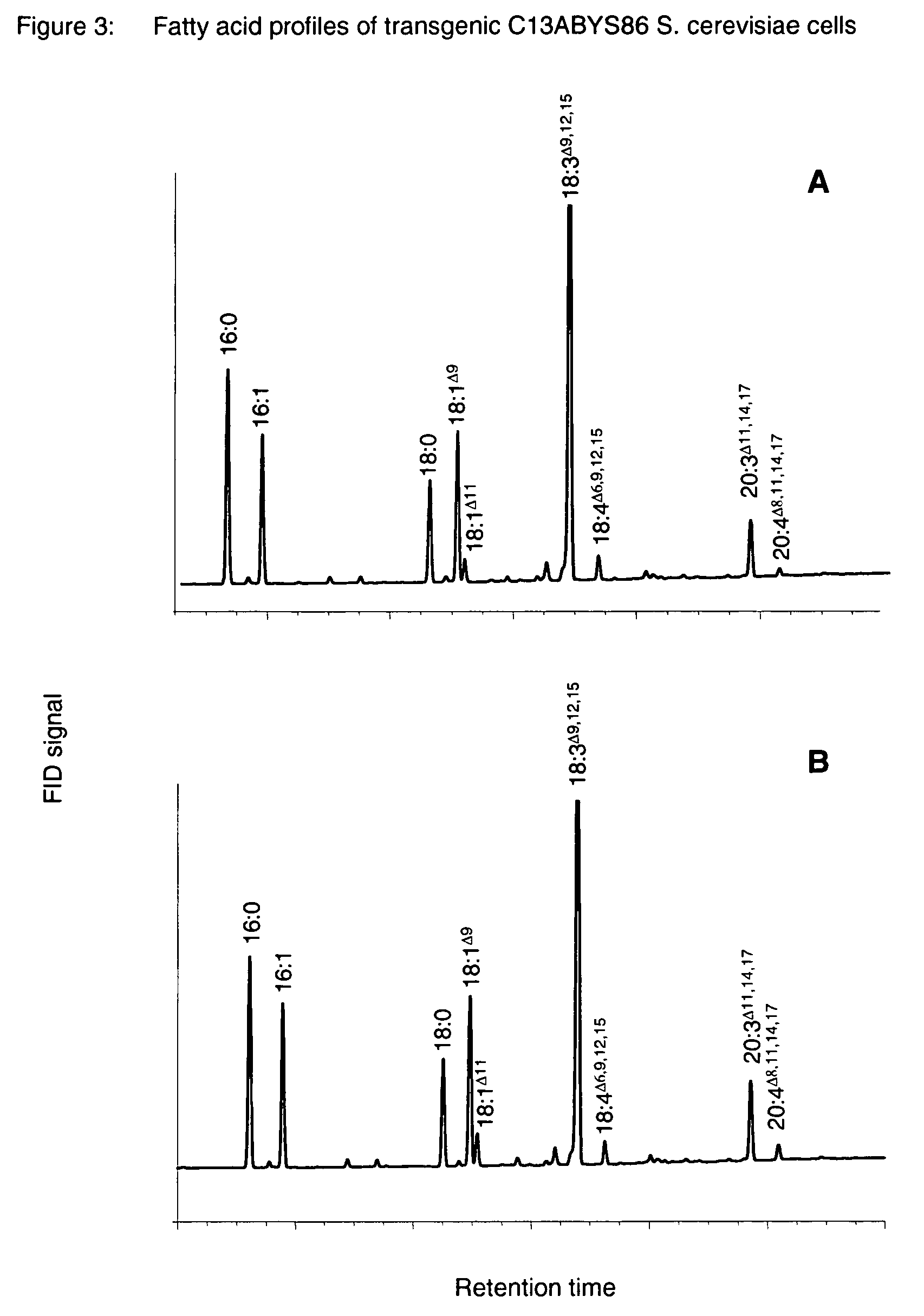
Method for the production of polyunsaturated fatty acids
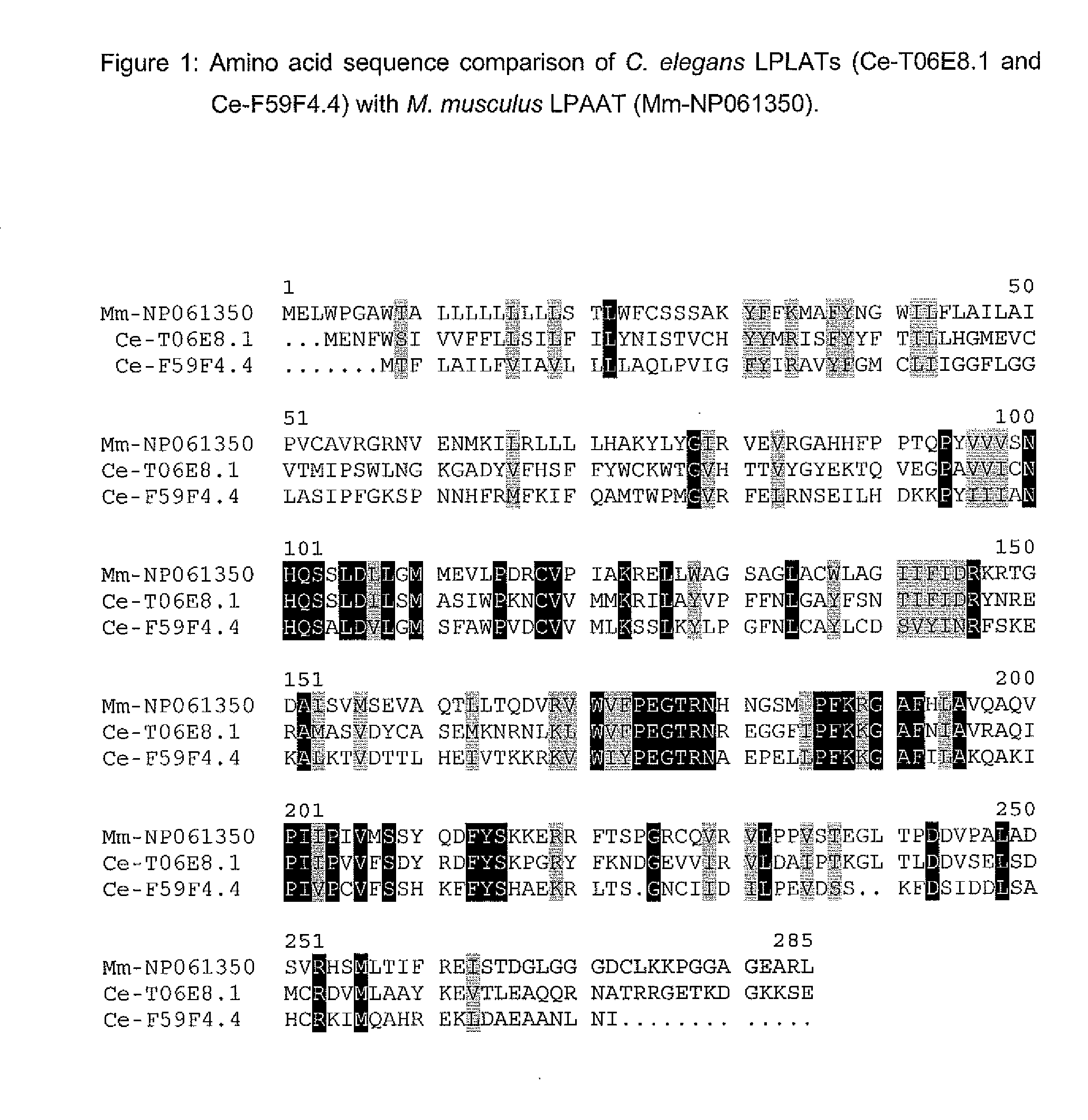
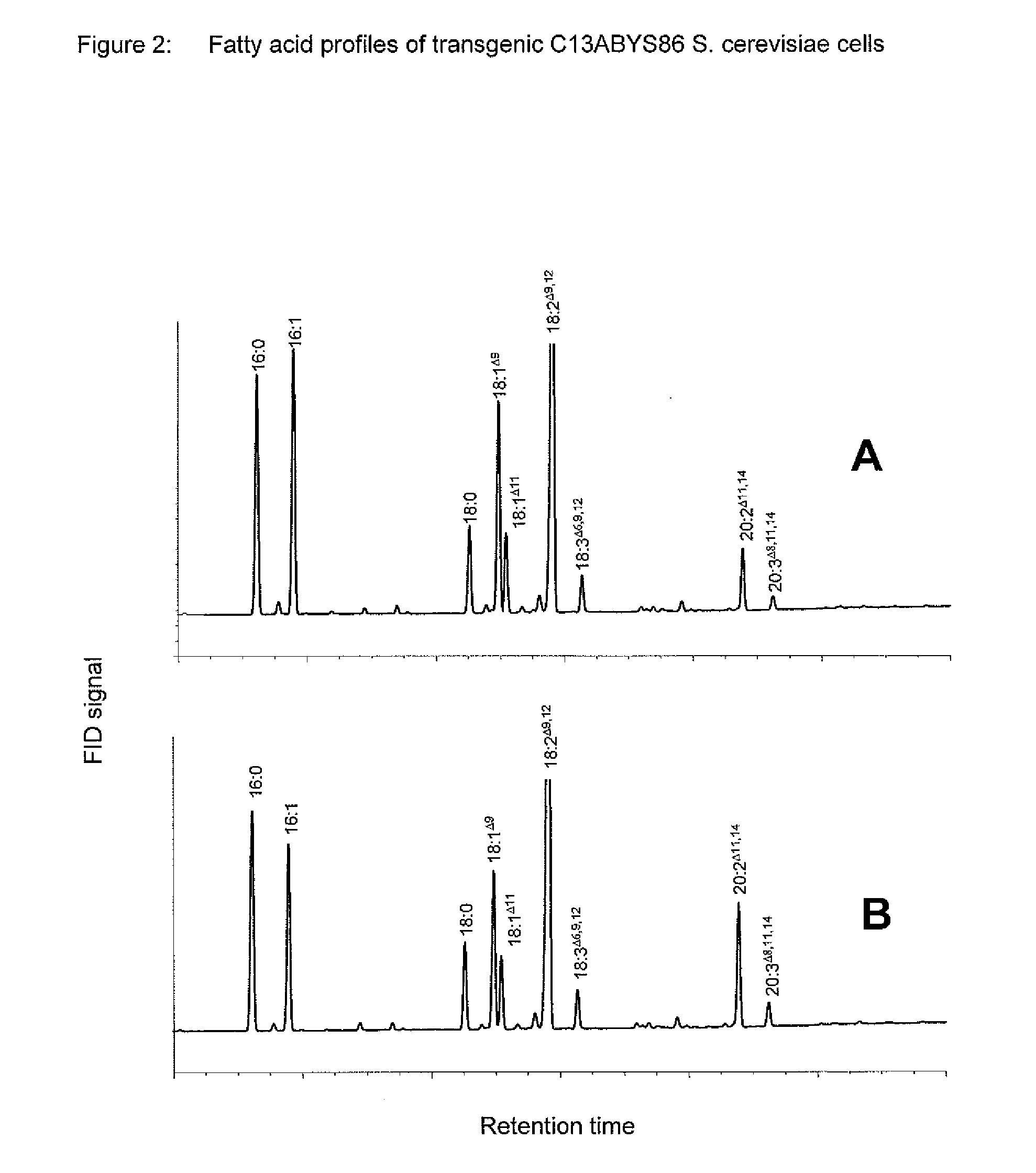
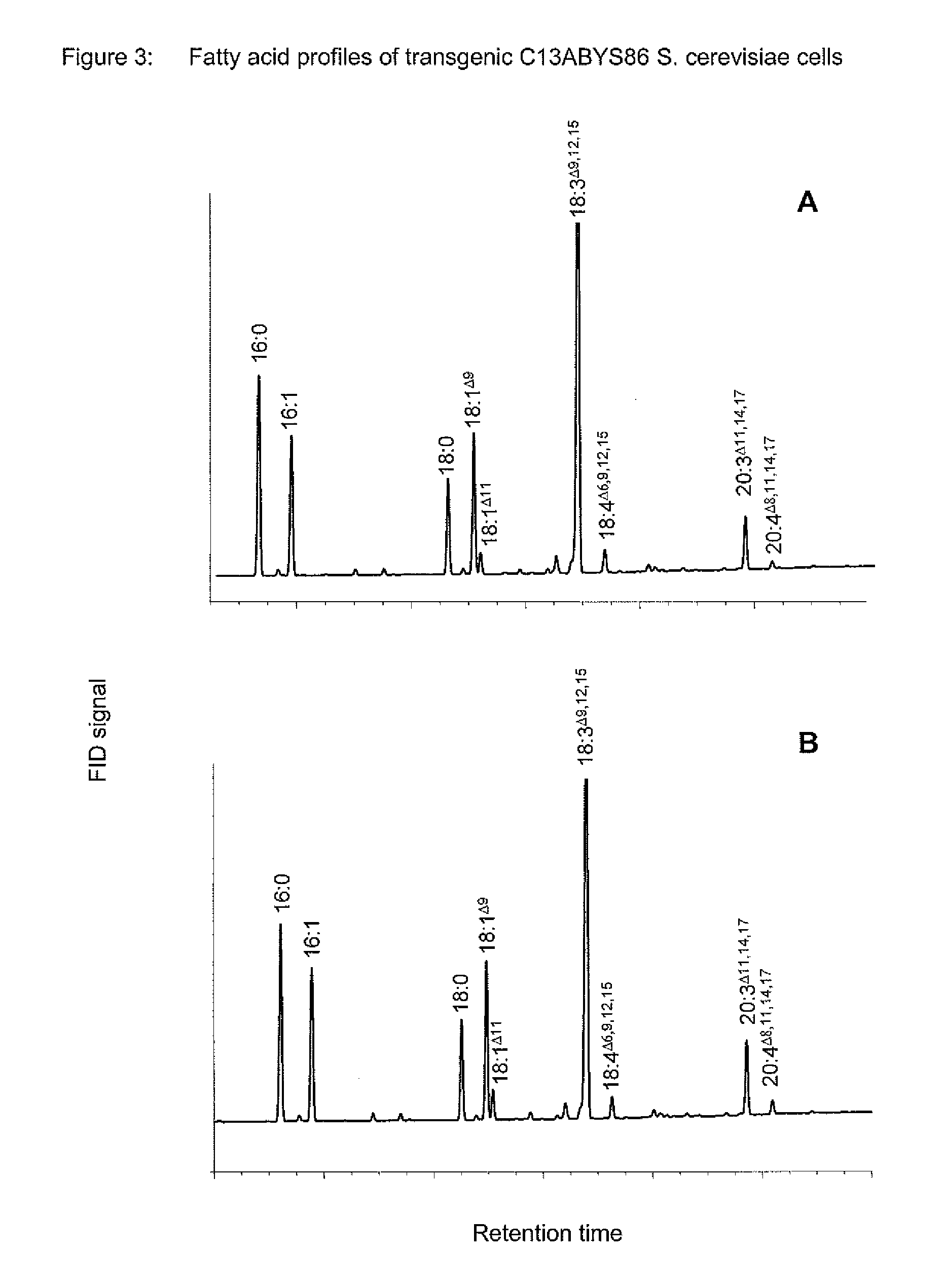
The present invention relates to a process for producing polyunsaturated fatty acids in an organism by introducing nucleic acids into the organism which code for polypeptides having acyl-CoA:lysophospholipid a cyltransferase activity. Advantageously, these nucleic acid sequences may, if appropriate together with further nucleic acid sequences coding for biosynthesis polypeptides of the fatty acid or lipid metabolism, be expressed in the transgenic organism. The invention furthermore relates to the nucleic acid sequences, to nucleic acid constructs comprising the nucleic acid sequences of the invention, to vectors comprising the nucleic acid sequences and / or the nucleic acid constructs and to transgenic organisms comprising the abovementioned nucleic acid sequences, nucleic acid constructs and / or vectors. A further part of the invention relates to oils, lipids and / or fatty acids produced by the process of the invention and to their use.
Owner:BASF PLANT SCI
Enzyme method for detecting disease conditions by measuring lysophosphatidic acid
InactiveUS6255063B1Reduce background concentrationMicrobiological testing/measurementBiological material analysisAssayLysophosphatidic acid
The present invention is an enzymatic method and diagnostic kits for detecting and quantifying the presence of one or more lysophospholids in a sample of bodily fluid taken from a test subject. The method uses enzymes in a two step assay and may be used to detect disease conditions associated with altered levels of lysophospholipids and to correlate such conditions with altered levels of lysophospholipids.
Owner:APOLLO ENDOSURGERY INC
Acyltransferase
InactiveUS7498026B2Fatty acid esters) is increasedEasy to calculateSugar derivativesPeptide/protein ingredientsNucleotideNucleotide sequencing
Owner:GENENCOR INT INC
Method for detecting cancer associated with elevated concentrations of lysophospholipids
InactiveUS20020123084A1Increases specificity and sensitivityImproved prognosisBiological testingOncologyTest subject
The present invention is methods for detecting the presence of cancer in a subject by determining the concentrations of lysophospholipids in a sample of bodily fluid taken from a test subject and comparing these concentrations to concentrations present in samples taken from normal subjects without cancer. The methods may be used for diagnosis and prognosis of cancer in a subject and to monitor the results of therapy of over time.
Owner:MILLS GORDON B +2
Engineering bacterium for producing Phospholipase A2 (PLA2) and applications thereof
InactiveCN102226165ANo activation requiredEasy to separate and purifyBacteriaFatty-oils/fats refiningVegetable oilA-DNA
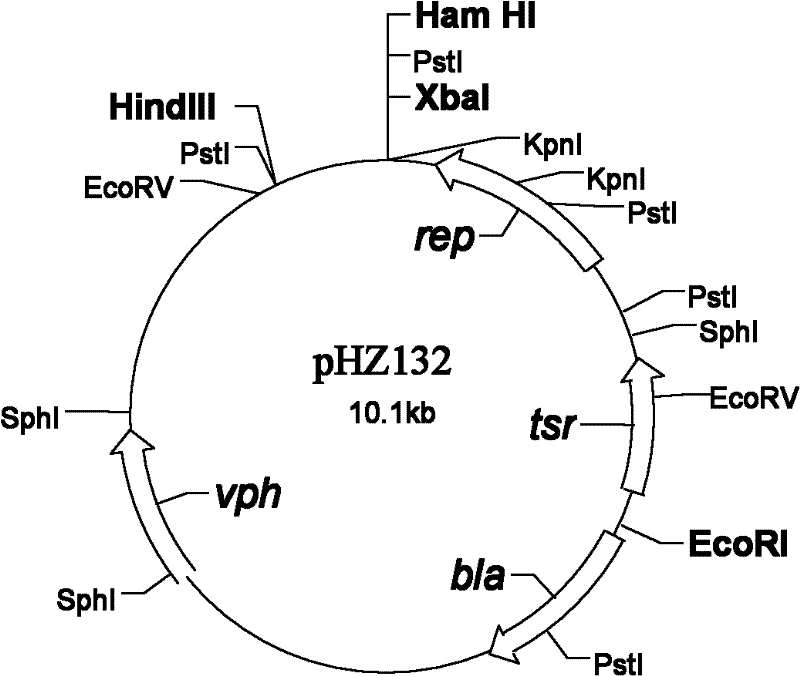
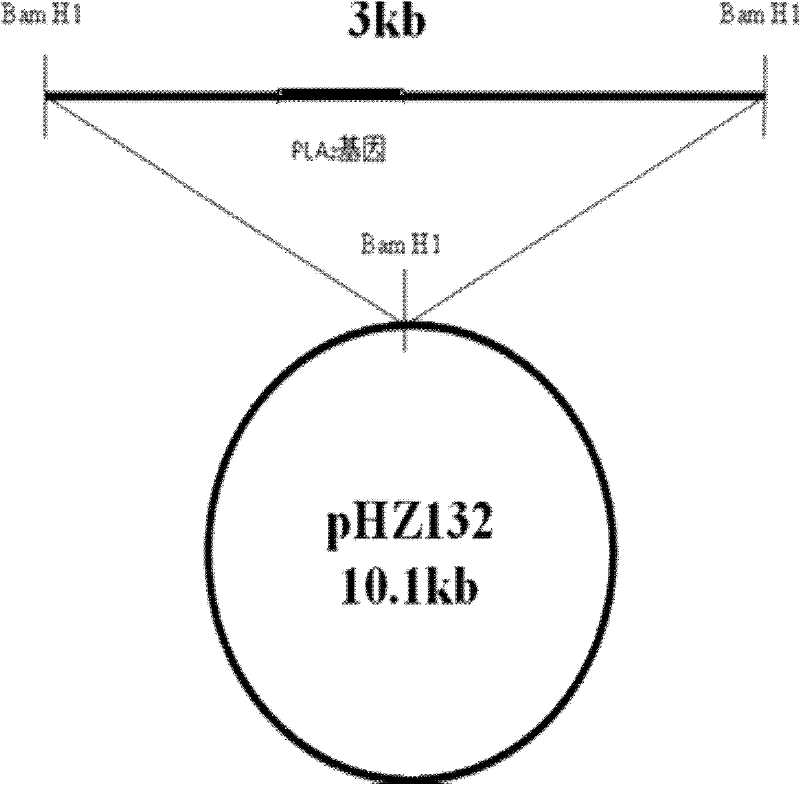
The invention discloses an engineering bacterium for producing a PL A2 and applications thereof. A preparation method of the PLA2 comprises the following steps: A, preparing a PLA2 gene: designing a primer PCR and amplifying a PLA2 gene fragment, and cloning a DNA fragment containing the PLA2 gene (a sequence is SEQ ID NO.2) with an amplified fragment plaT as a probe; B, constructing a recombinant plasmid: cloning a Bam H1 restricted fragment with the size of 3 kb which contains the PLA2 gene to a high-copy plasmid pHZ132 to obtain a recombinant plasmid pLH 001; and C, constructing the engineering bacterium: introducing the plasmid pLH001 into a streptomyces lividans 1326 of a host bacterium through protoplast transformation to obtain a streptomyces lividans LH001 of the engineering bacterium. The engineering bacterium has good stability and the introduced plasmid is not easy to lose. The secretory active PLA2 (an amino acid sequence is SEQ ID NO.1) can be generated through liquid fermentation, the enzymatic activity of a fermentation liquid is equal to or more than 2,000 U / mL, and the enzyme production capability is better than present levels. The PLA2 can be applied to fields of vegetable oil degumming, lysophospholipid preparation and the like.
Owner:HUAZHONG AGRI UNIV
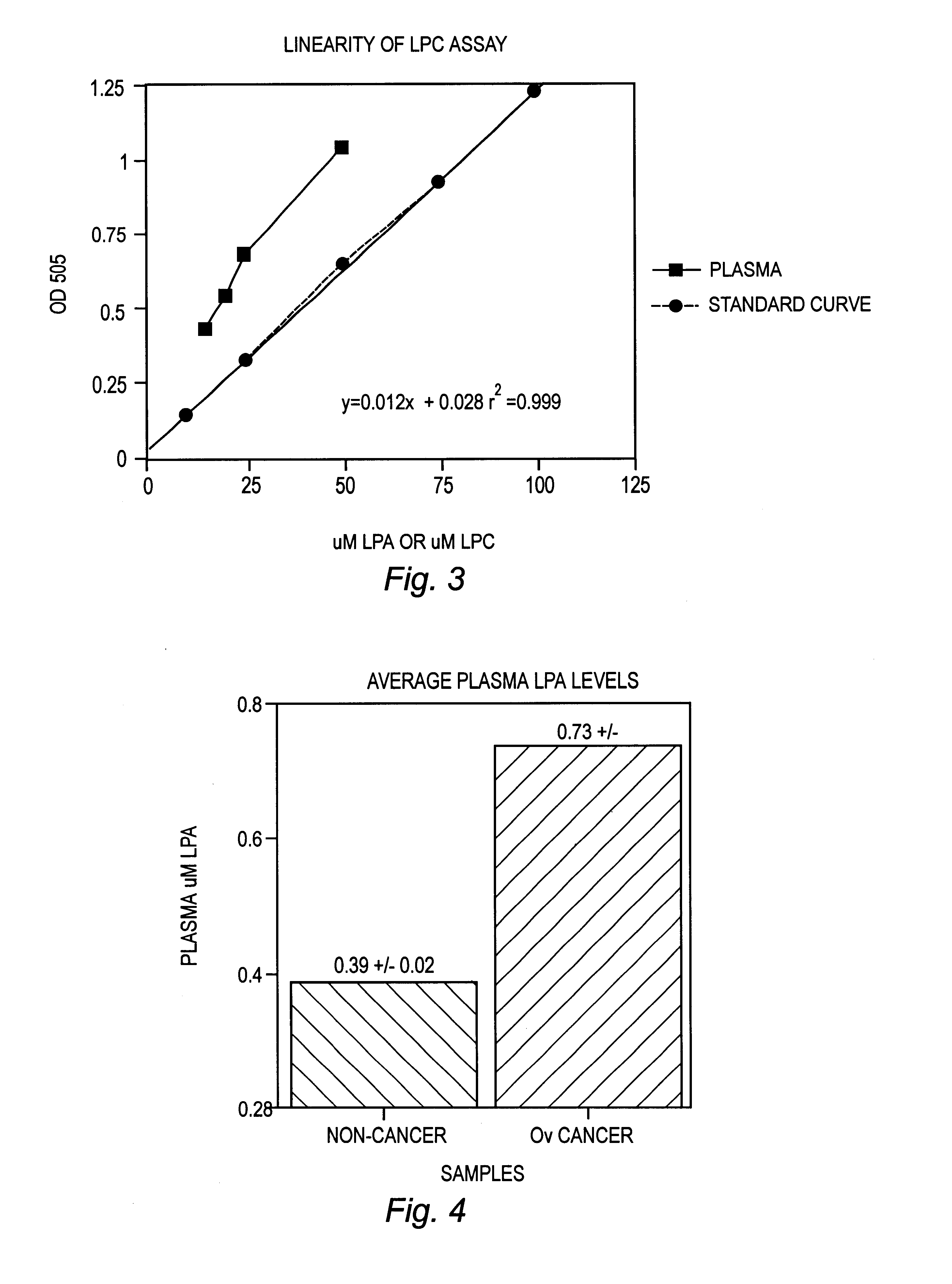
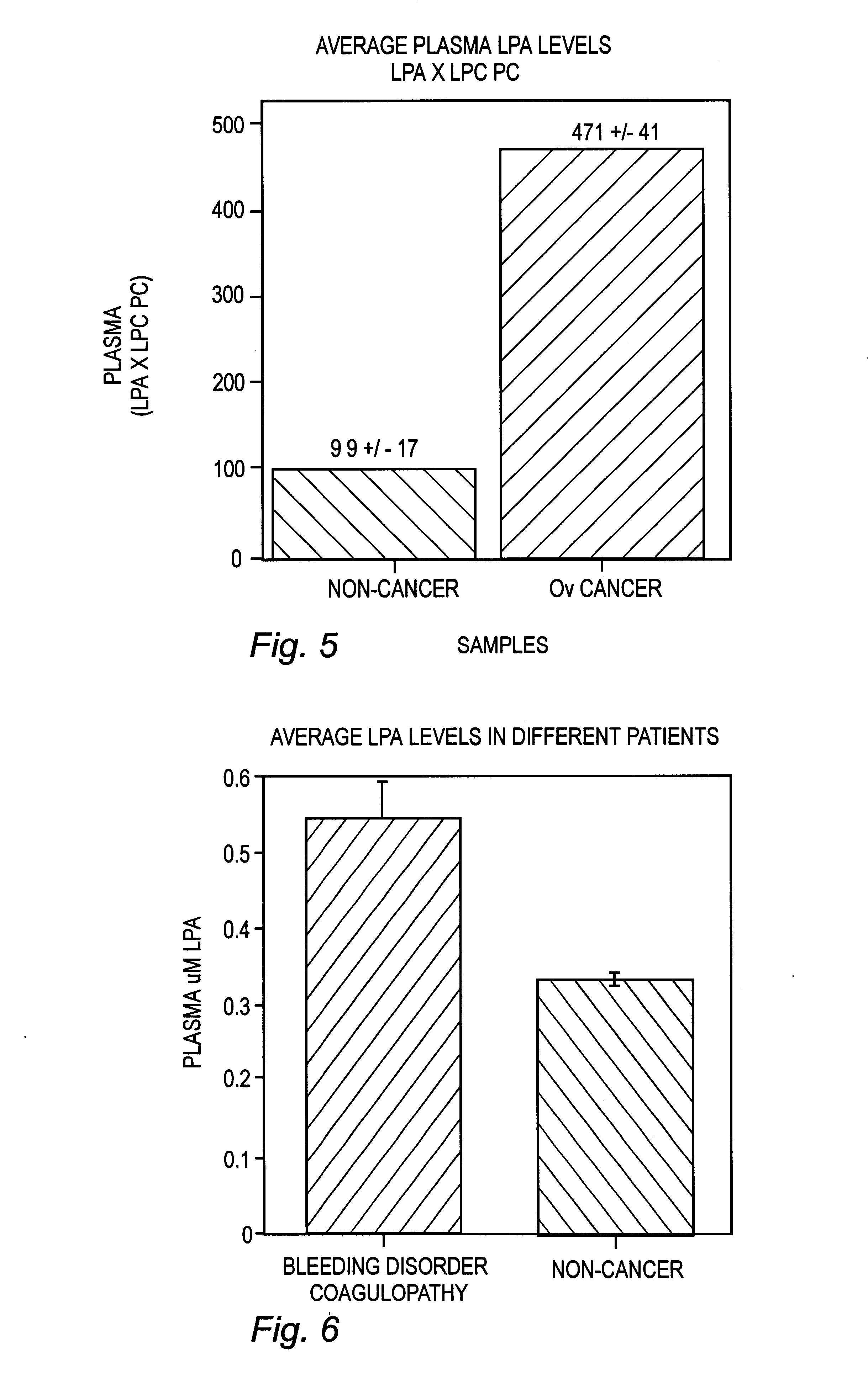
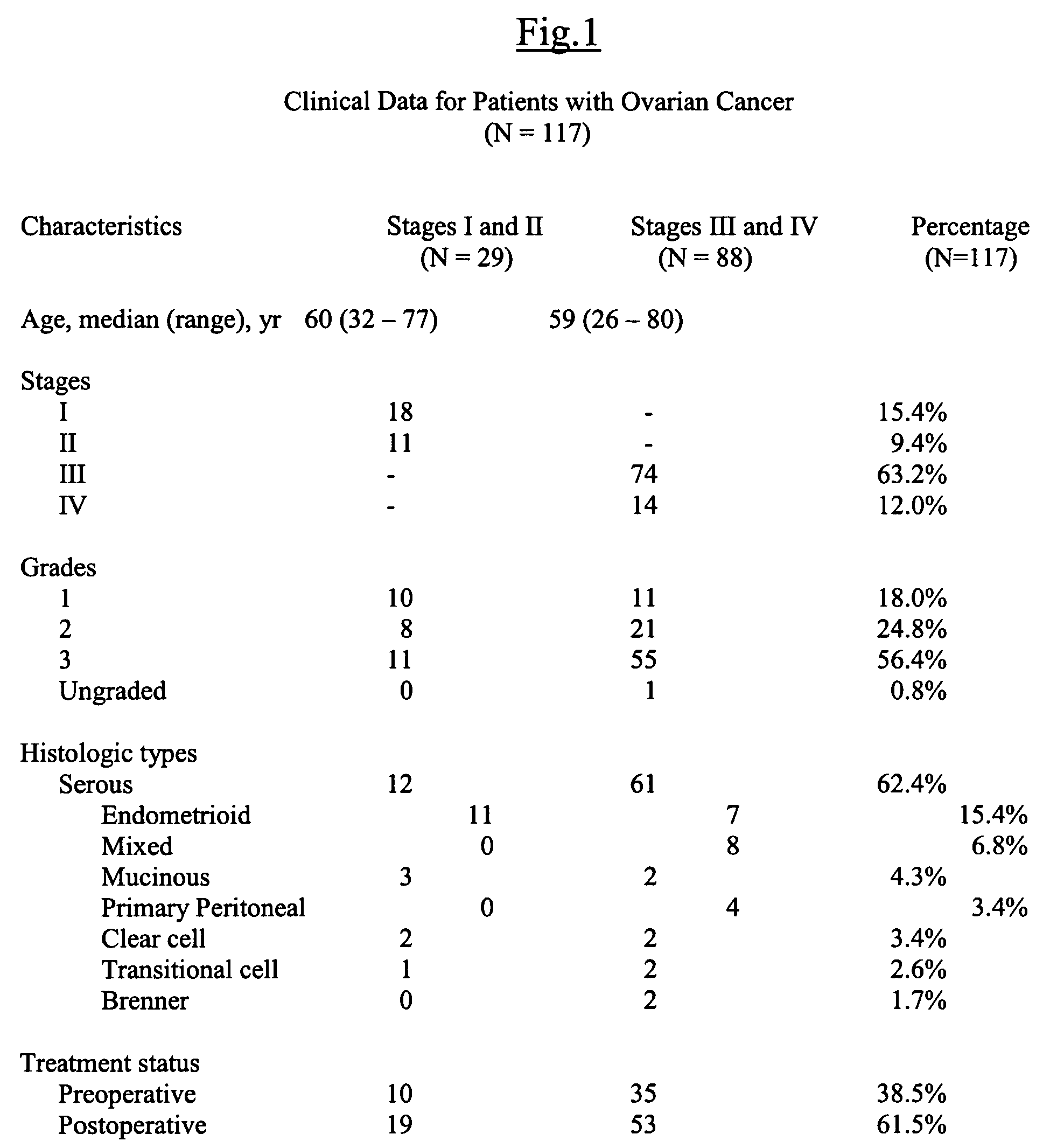
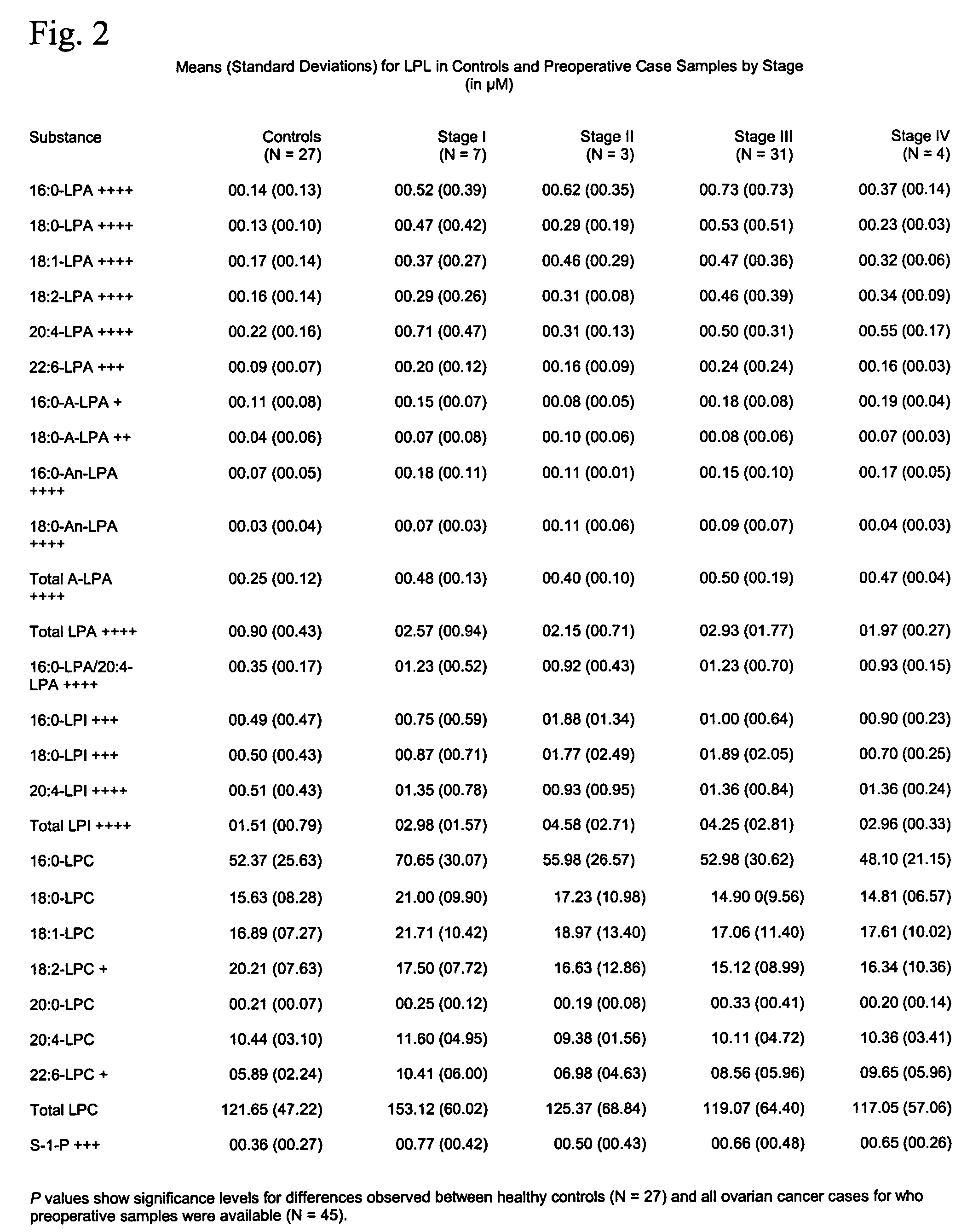
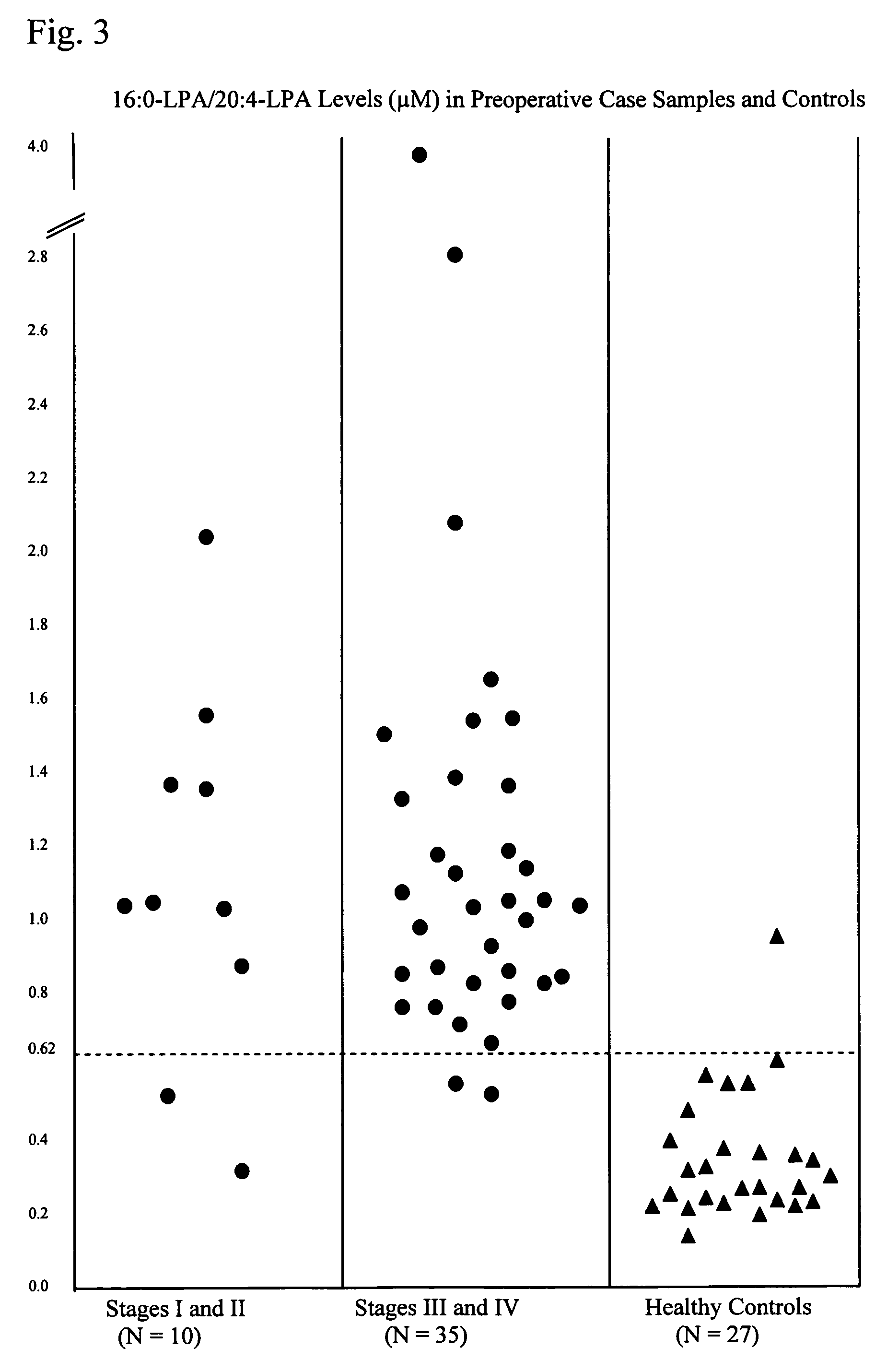
Lysophospholipids as biomarkers of ovarian cancer
InactiveUS7964408B1Improve survivalIncreased mortalityMicrobiological testing/measurementMass spectrometric analysisPhosphateSubspecies
A method of using a bioactive lysophospholipid (LL) as a biomarker for detecting the presence and recurrence of ovarian cancer. Subspecies of LL, such as lysophosphatidic acid (LPA), lysophosphatidylinositol (LPI), lysophosphatidylcholine (LPC), and lysosphingolipid sphinsosine-1-phosphate (S1P), are used alone or in conjunction to increase the specificity and sensitivity of the assay.
Owner:UNIV OF SOUTH FLORIDA
Method for preparing solubilized composition containing oil-soluble substance
ActiveUS20080070992A1Improve acid resistanceImprove heat resistanceBiocideCosmetic preparationsSucroseEthyl acetate
The present invention provides a method for preparing a solubilized composition containing an oil-soluble substance having both acid and heat resistance, including:the step of dissolving an oil-soluble substance and two or three emulsifiers selected from(1) an emulsifier E1 comprising an ester of a fatty acid having an HLB of not less than 10 and not more than 14 carbon atoms with a polyglycerol having a polymerization degree of not less than 3,(2) an emulsifier E2 comprising an ester of a fatty acid having an HLB of not less than 10 and not more than 14 carbon atoms with sucrose, or(3) an emulsifier E3 comprising lecithin in which phosphatidylcholine accounts for not less than 50% and / or lysolecithin in which lysophosphatidylcholine accounts for not less than 50% of a phospholipid content in (a) ethanol or (b) a mixed solvent of ethanol with at least one selected from the group consisting of acetone, hexane, and ethyl acetate to prepare a transparent solution; andthe step of distilling the solvent off from the transparent solution.
Owner:TSUJI SEIYU
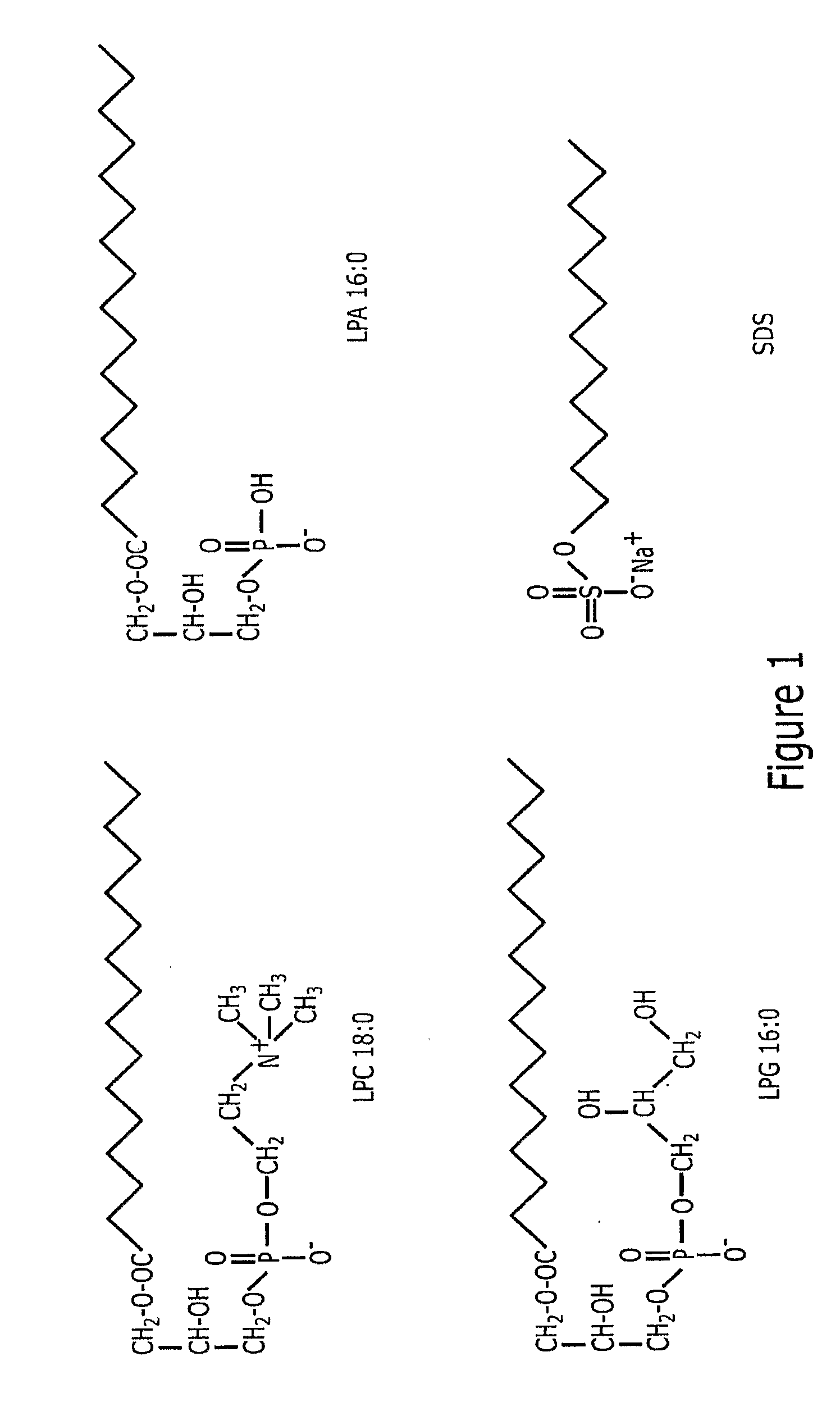
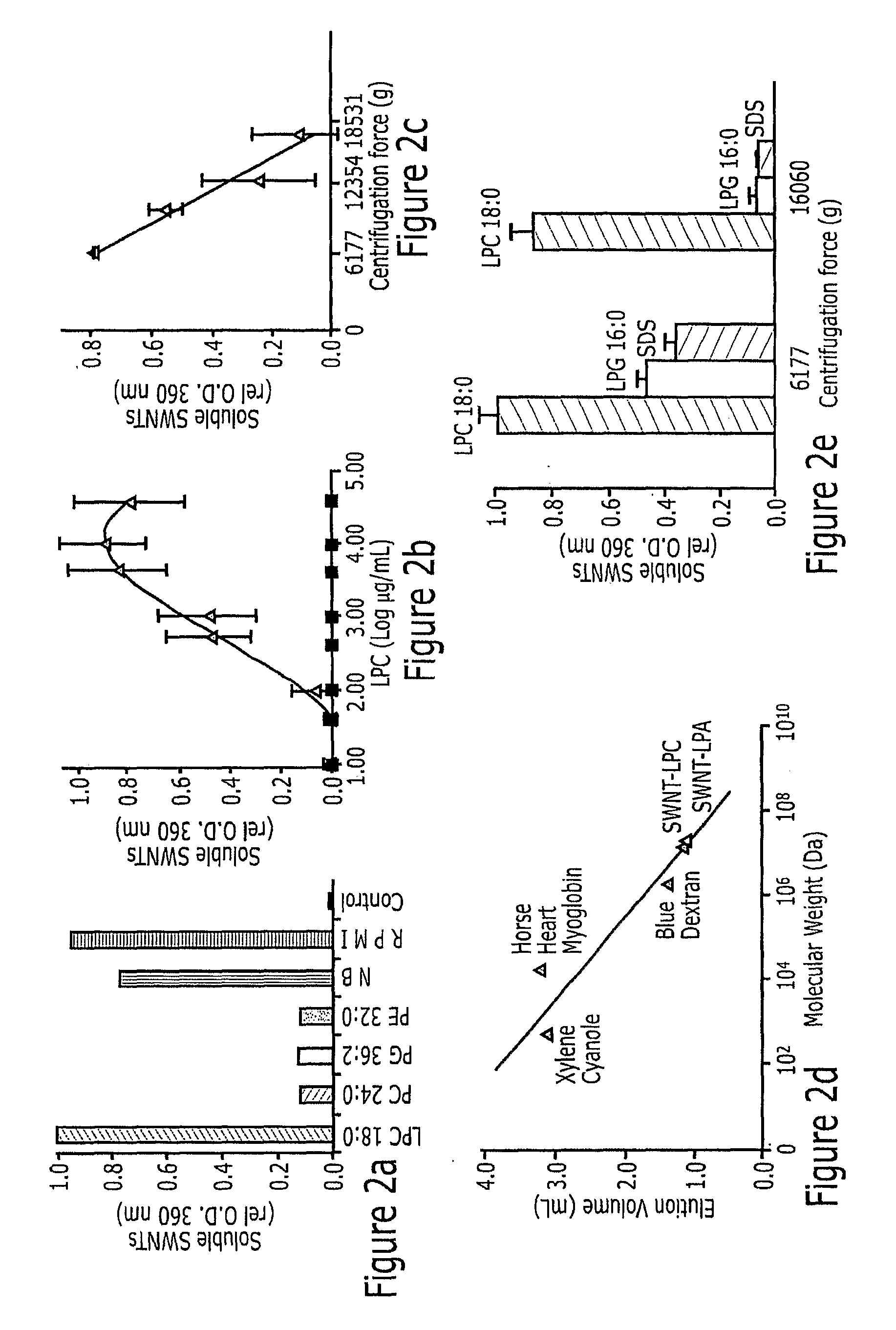
Lysophospholipids Solubilized Single-Walled Carbon Nanotubes
InactiveUS20090162277A1Improve solubilityLong-term stabilityPowder deliveryMicrobiological testing/measurementMedicineBiocompatibility Testing
Lipophilic compounds extracted from cell growth mediums, particularly lysophospholipids are used to solubilize single-walled nanotubes. The naturally occurring lysophospholipids were found to readily bond to the exterior wall of the single-walled nanotubes to enhance the biocompatibility of the single-walled nanotubes in therapeutic and diagnostic conditions. The solubilization protocol is simple, highly efficient, and results in a population of coated single-walled nanotubes which are highly stable.
Owner:CLEMSON UNIV RES FOUND
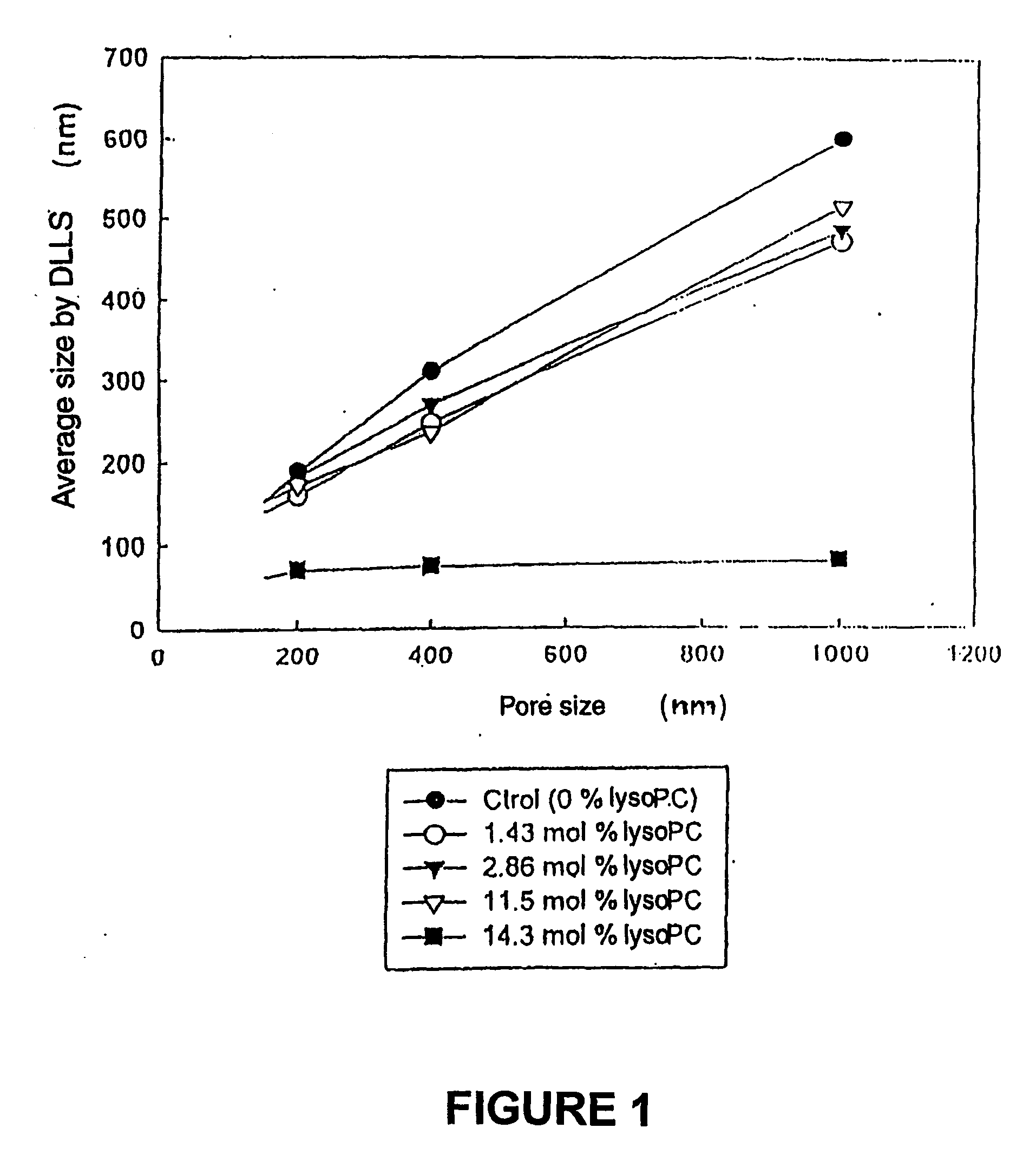
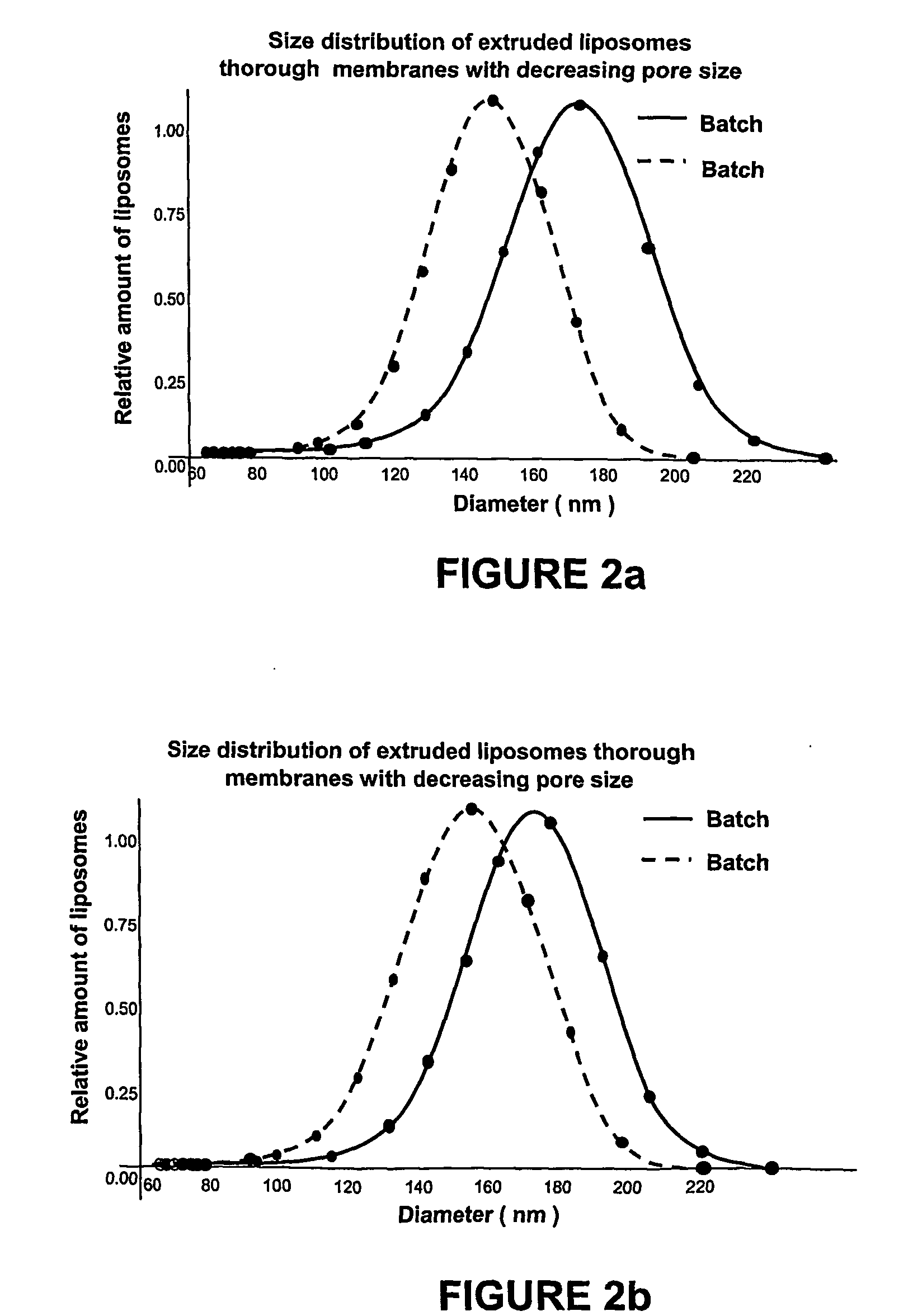
Pharmaceutical composition of small-sized liposomes and method of preparation
InactiveUS20060078605A1Simple procedureIncrease volumeBiocideCarbohydrate active ingredientsLipid formationAtherion elymus
A pharmaceutical composition of small sized unilamellar liposomes for the supply active principles by injection, with an improved permanency in the blood flow, where the unilamellar membrane contains a mixture of saturated lipids encompassing at least one lysophospholipid in a quantity from about 0.5 mol % to about 6.0 mol % with reference total lipids and the production method. Additionally, liposomes of high encapsulation efficiency of an active principle like doxorubicine are prepared through the adding of a solution of calcium ions.
Owner:MAMMARELLA CARLOS ALBERTO GENARO
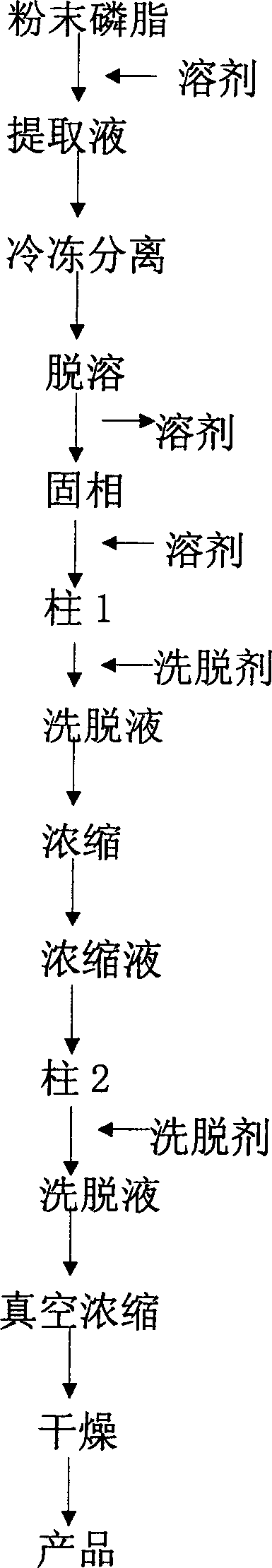
Process for preparing high purity soy phoshatidylcholine without lysophosphatide
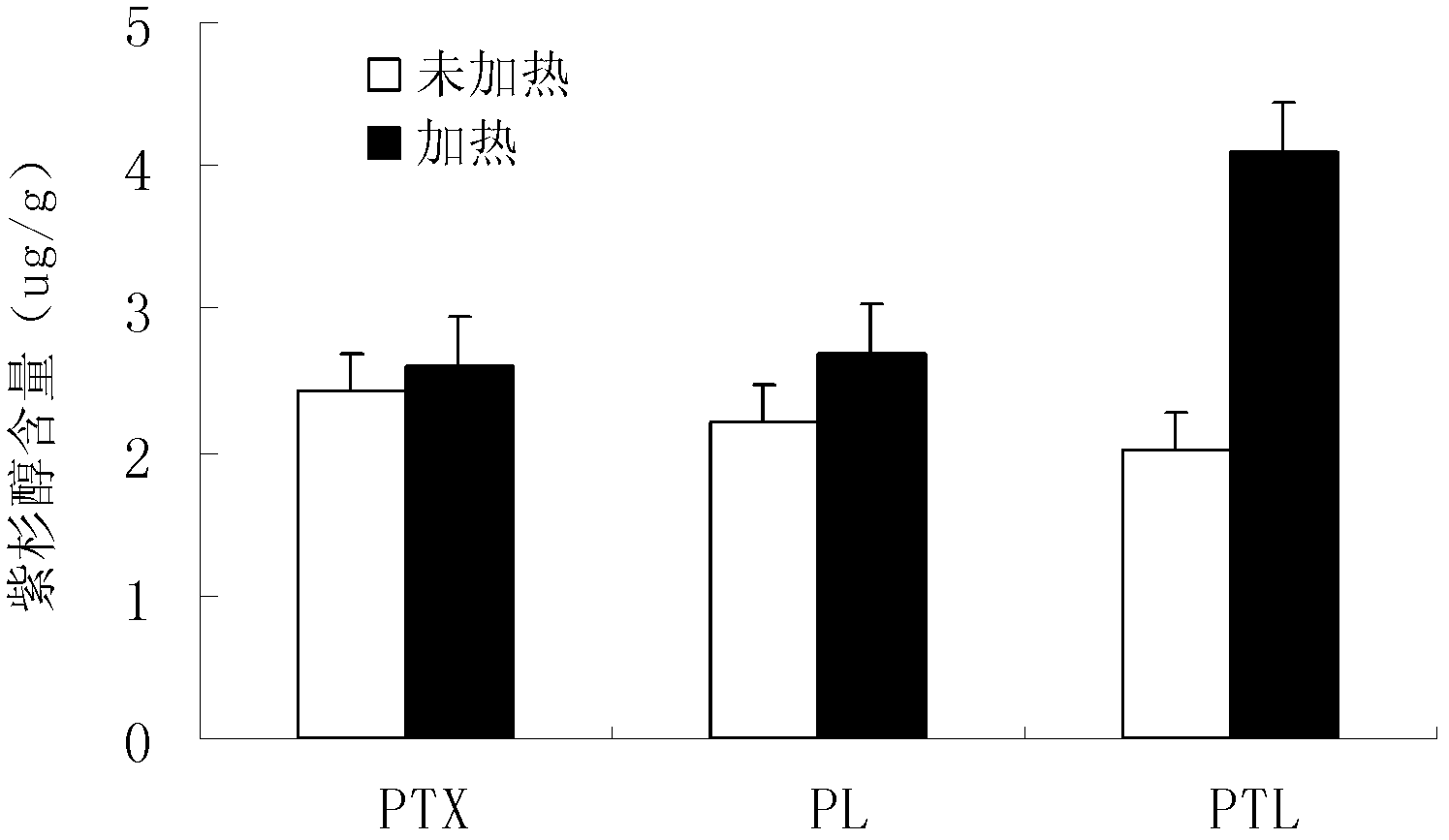
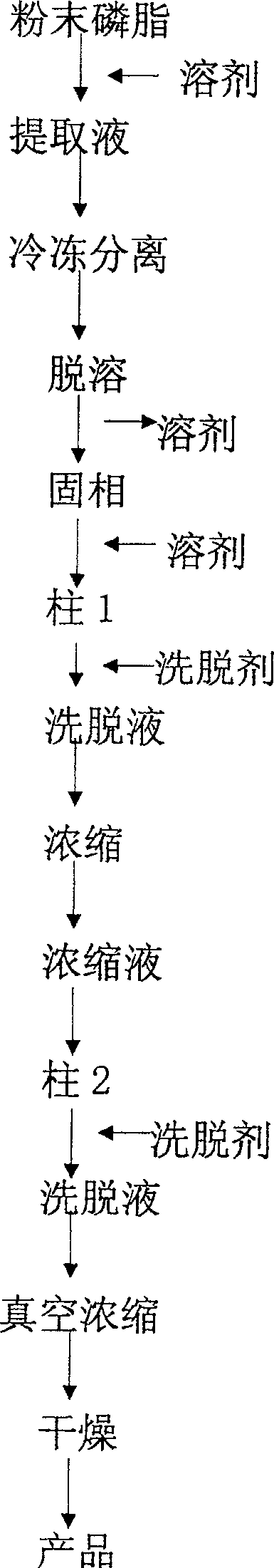
InactiveCN1837222AEasy to manufactureWide range of usesPhosphatide foodstuff compositionsSolventSoybean Phospholipids
The invention discloses a high-purity soya bean phosphatidyl choline without lysophosphatide preparing method in the phosphatide technique domain, which comprises the following steps: using soybean podwer phosphatide for raw material; extracting by dissolvant; freezing raffinate; treating liquor by dual column chromatogram; eluting for treating column 1; dislodging phosphatidyl ethanolamine; eluting and treating column2; dislodging lysophosphatide, wherein eluent of dissolvant,column 1 and column 2 for extracting is common single solvent; the production has no lysophosphatide; the bilineurine content of soya bean lecithin is more than 90%.
Owner:JIANGNAN UNIV
Use of Lysophospholipids to Treat Inflammation
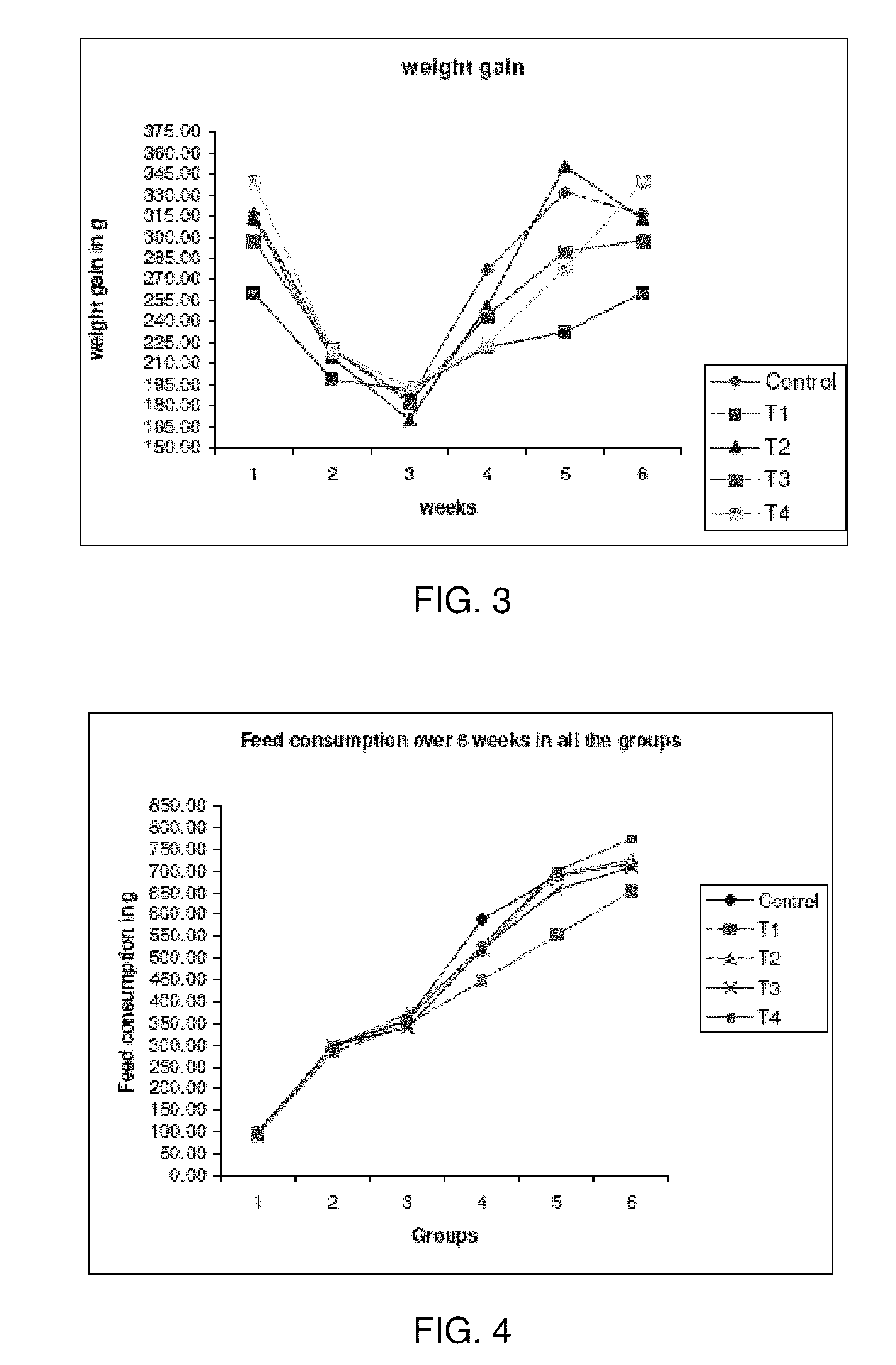
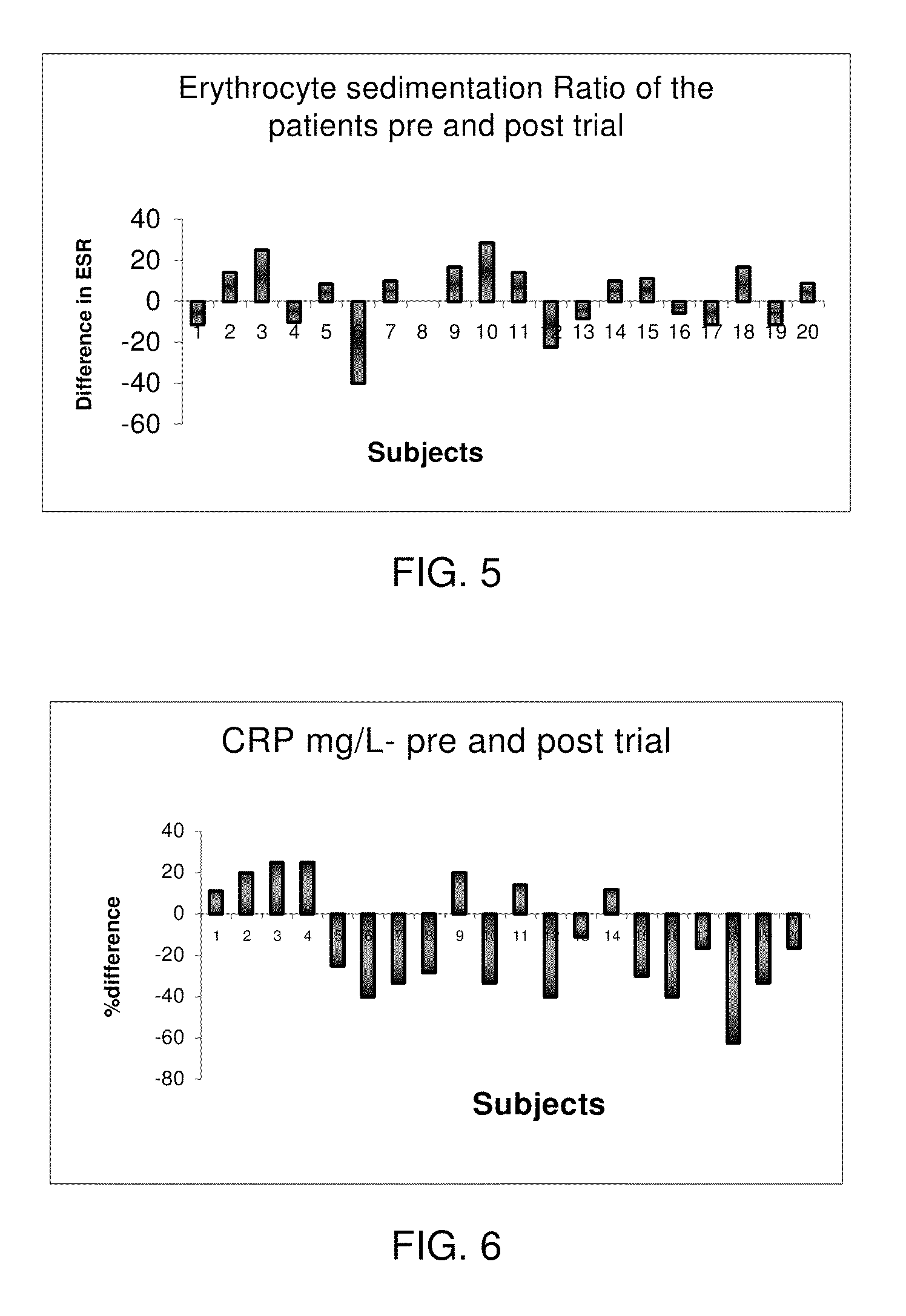
InactiveUS20090281065A1Improve feed conversion rateIncrease weight gainBiocideAntipyreticPsychiatryInflammation
Phospholipids and lysophospholipids, more particular lysophospatidylcholine (LPC), known to be pro-inflammatory in certain amounts, are demonstrated to be useful in the prevention, the treatment or amelioration of the effects of inflammation.
Owner:KEMIN IND INC
Instant yolk powder and preparation method thereof
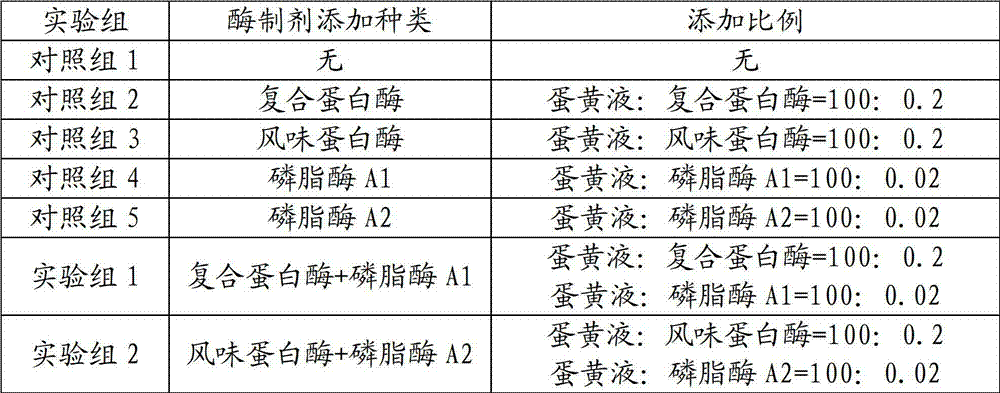
The invention provides a preparation method of instant yolk powder. The method comprises the following steps of: (a) mechanically dispersing yolk solution; (b) adding protease and phospholipase to the yolk solution, wherein the weight ratio of yolk solution to protease is 100:(0.1-0.5) and the weight measurement ratio of yolk solution to phospholipase is 100:(0.01-0.05); and (c) carrying out drying treatment. The preparation method has the beneficial effects that the proteins are appropriately hydrolyzed to micromolecules with different chain lengths by adding the protease and simultaneously the phospholipase is added to appropriately hydrolyze phospholipid in the yolk solution to generate lysophospholipid; and through synergy among enzyme preparations, such function properties of the hydrolysate as solubility, emulsibility and heat resistance are more obvious.
Owner:北京二商健力食品科技有限公司
Generation of Triacylglycerols from Gums
ActiveUS20090173689A1Fatty oils/acids recovery from wasteFatty acid esterificationPhosphateFatty acid
A method is disclosed for the generation of triacylglycerols from gums that have been separated from an oil product. The gums are treated with an enzyme having PLC activity, which results in the formation of diacylglycerols and phosphates, and treated with an enzyme having PLA activity, which results in the formation of lyso-phospholipids and free fatty acids. The diacylglycerols and the free fatty acids from these two separate reactions then combine in the presence of the enzymes to generate new triacylglycerol molecules.
Owner:BUNGE OILS INC
Use of lysophosphatidylethanolamine (18:1) and lysophosphatidylinositol to retard senescence and to enhance fruit ripening
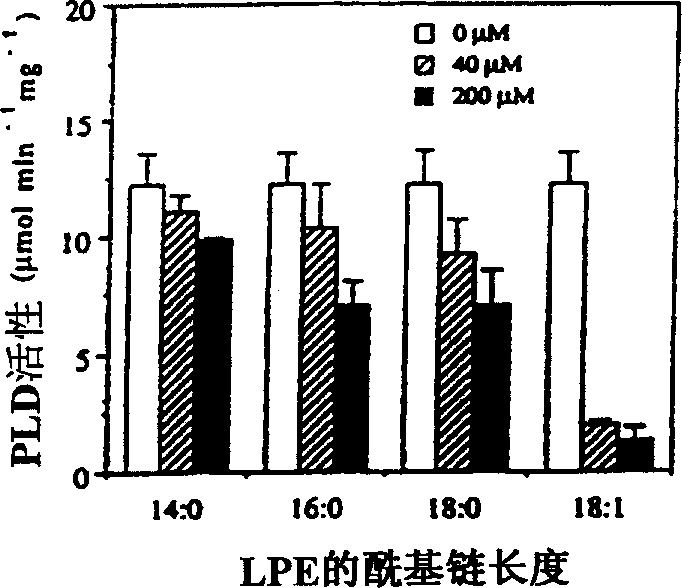
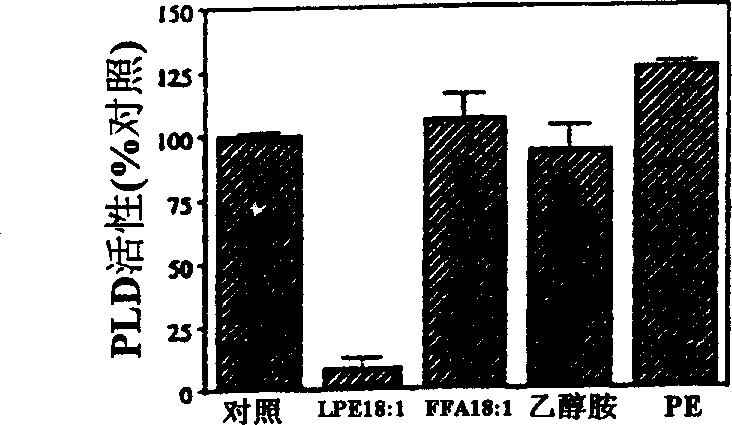
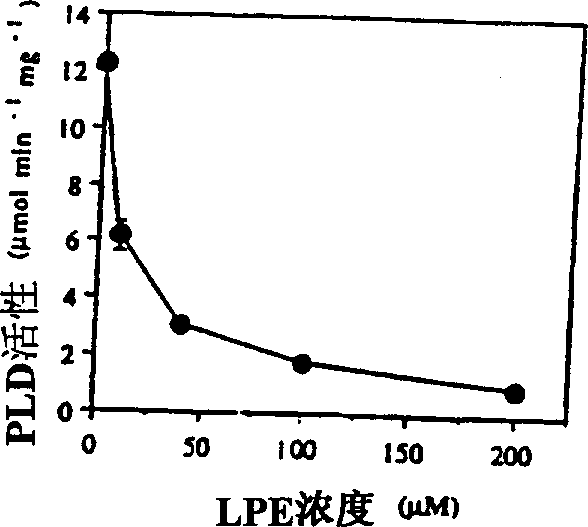
The present invention relates to a method of enhancing fruit ripening and stability and of delaying senescence in fruit and other plant tissues. This method consists of applying an effective amount of a lysophospholipid, such as lysophosphatidylethanolamine (18:1) (hereinafter referred to as LPE (18:1)) or lysophosphatidylinositol (hereinafter referred to as LPI) to the fruit and other plant tissues. Lysophospholipids such as LPE (18:1) and LPI were found to be superior to other lysophospholipids in delaying senescence and in inhibiting phospholipase D, a key enzyme in mediating membrane deterioration during of plant senescence. LPE (18:1) and LPI are naturally occurring and environmentally safe. Their use could replace many environmentally toxic compounds that are currently being used to retard senescence of flowers, fruits and leaves and to enhance fruit ripening.
Owner:WISCONSIN ALUMNI RES FOUND
Applications of temperature sensitive composite liposome in controlled release of water soluble and amphiphilic anti-cancer drugs
InactiveCN104721137AIncrease temperatureImprove stabilityEnergy modified materialsPharmaceutical non-active ingredientsLipid formationBiocompatibility Testing
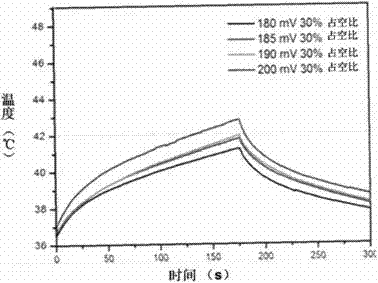
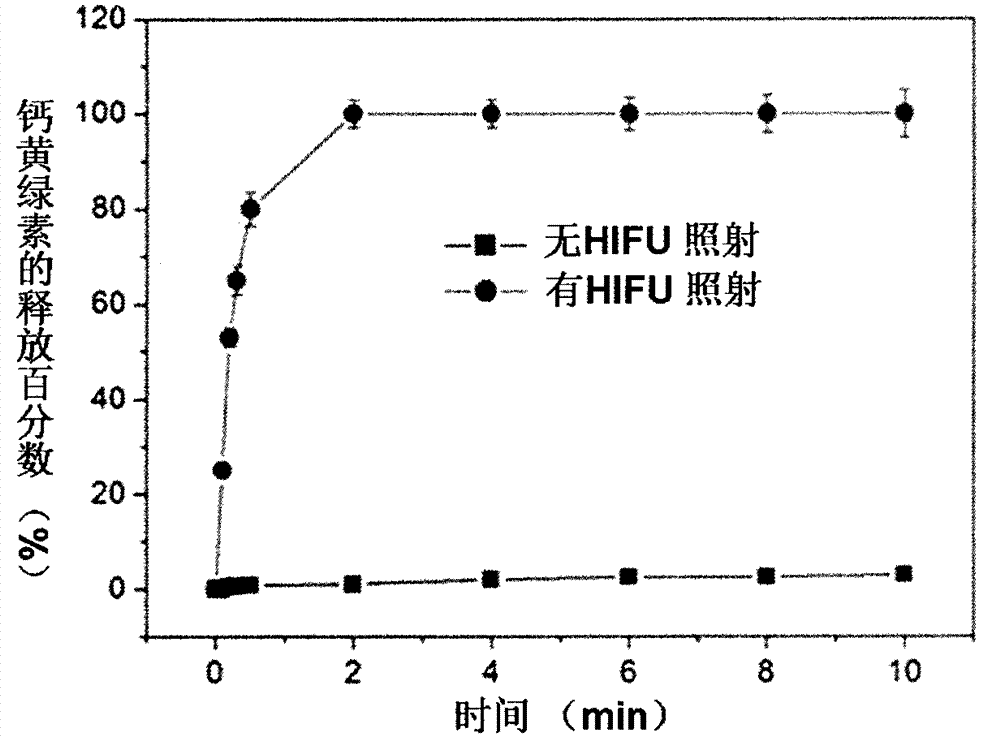
The invention discloses a composite liposome sensitive to high-intensity focused ultrasound (HIFU) and temperature, and applications thereof. The composite liposome is taken as a carrier used for drug intelligent controlled release under HIFU or heating. The composite liposome comprises at least one composite liposome forming lipid (CFL), at least one temperature-sensitive phospholipid, at least one lysophospholipid, and a lipid with covalently connected poly (ethylene glycol) (PEG). Temperature sensitive range of the composite liposome ranges from 39.0 to 45.0 DEG C; cyclicity is long; and the composite liposome is sensitive to HIFU. The invention also relates to applications of the HIFU and temperature sensitive composite liposome in controlled release of water soluble and amphiphilic anti-cancer drugs. The temperature sensitive composite liposome nanoparticle possesses excellent stability and biocompatibility, and excellent responsiveness capability on HIFU; under HIFU irradiation, the water soluble and amphiphilic anti-cancer drugs can be released out from the temperature sensitive composite liposome quickly; and curative effect is improved substantially.
Owner:PEKING UNIV
Cloned human lysophospholipase
InactiveUS7294496B1Inhibition is irreversibleImprove the level ofSugar derivativesBacteriaDiseaseBinding site
A cloned of a human brain lysophospholipid-specific lysophospholipase enzyme molecule, its potential use for treatment of a host of diseases and method of inactivation are disclosed. Also disclosed are its distribution in tissue sand detailed kinetic analysis. hLysoPLA has a single substrate binding site and a surface recognition site. In contrast to many nonspecific lipolytic enzymes that exhibit lysophospholipase activity, hLysoPLA hydrolyzes only lysophospholipids and has no other significant enzymatic activity.
Owner:RGT UNIV OF CALIFORNIA
Compositions And Methods For Neovascularization
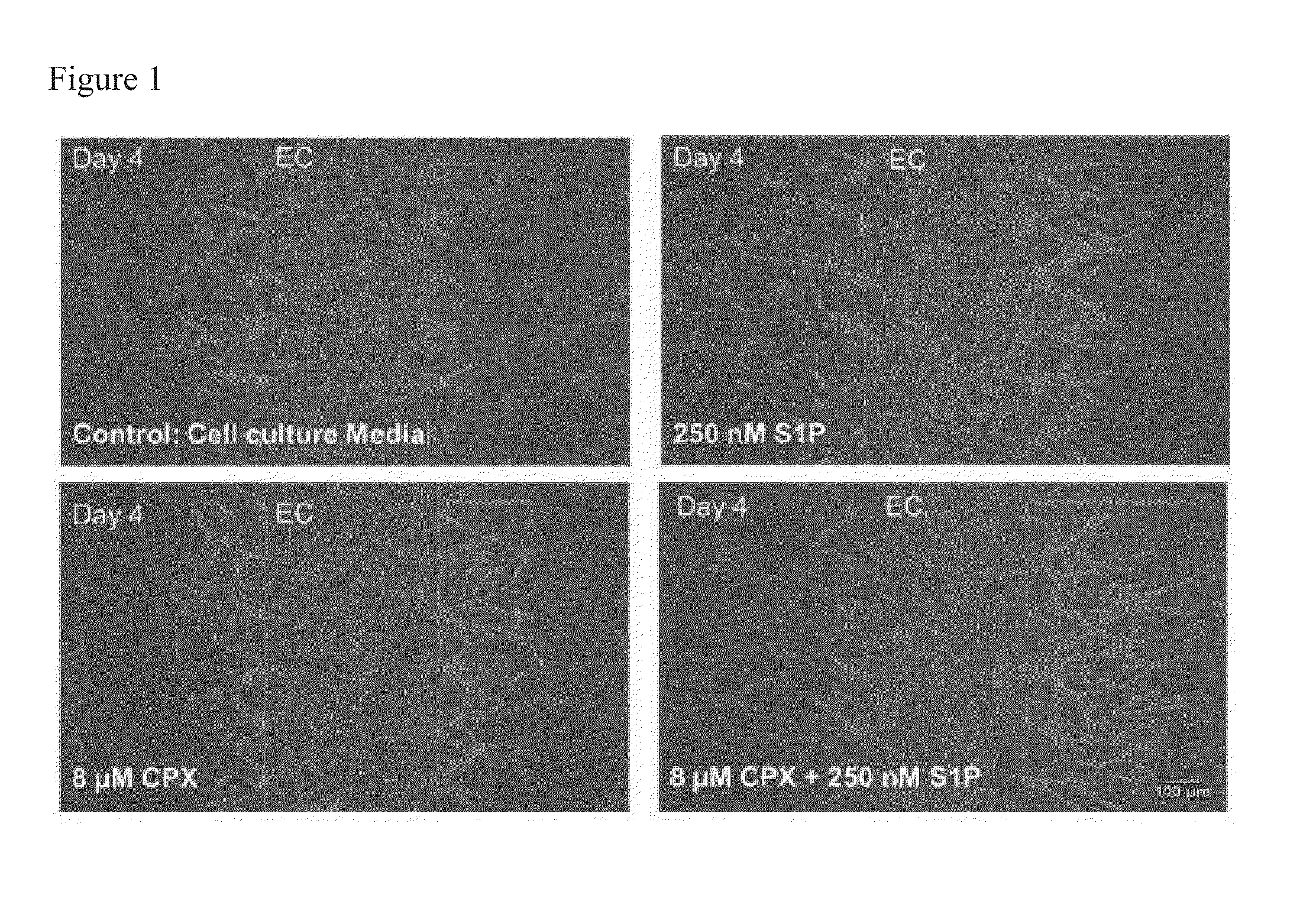
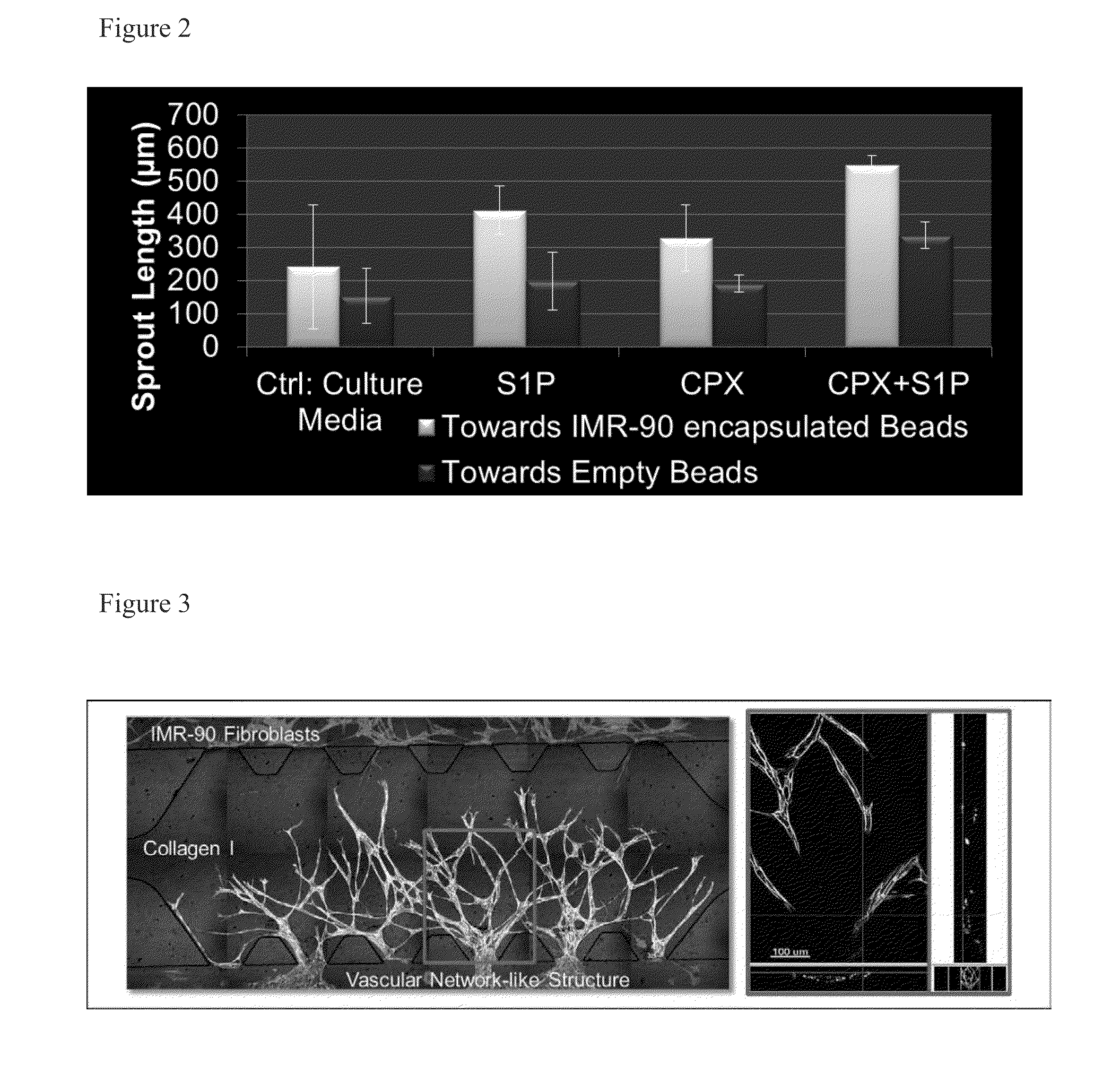
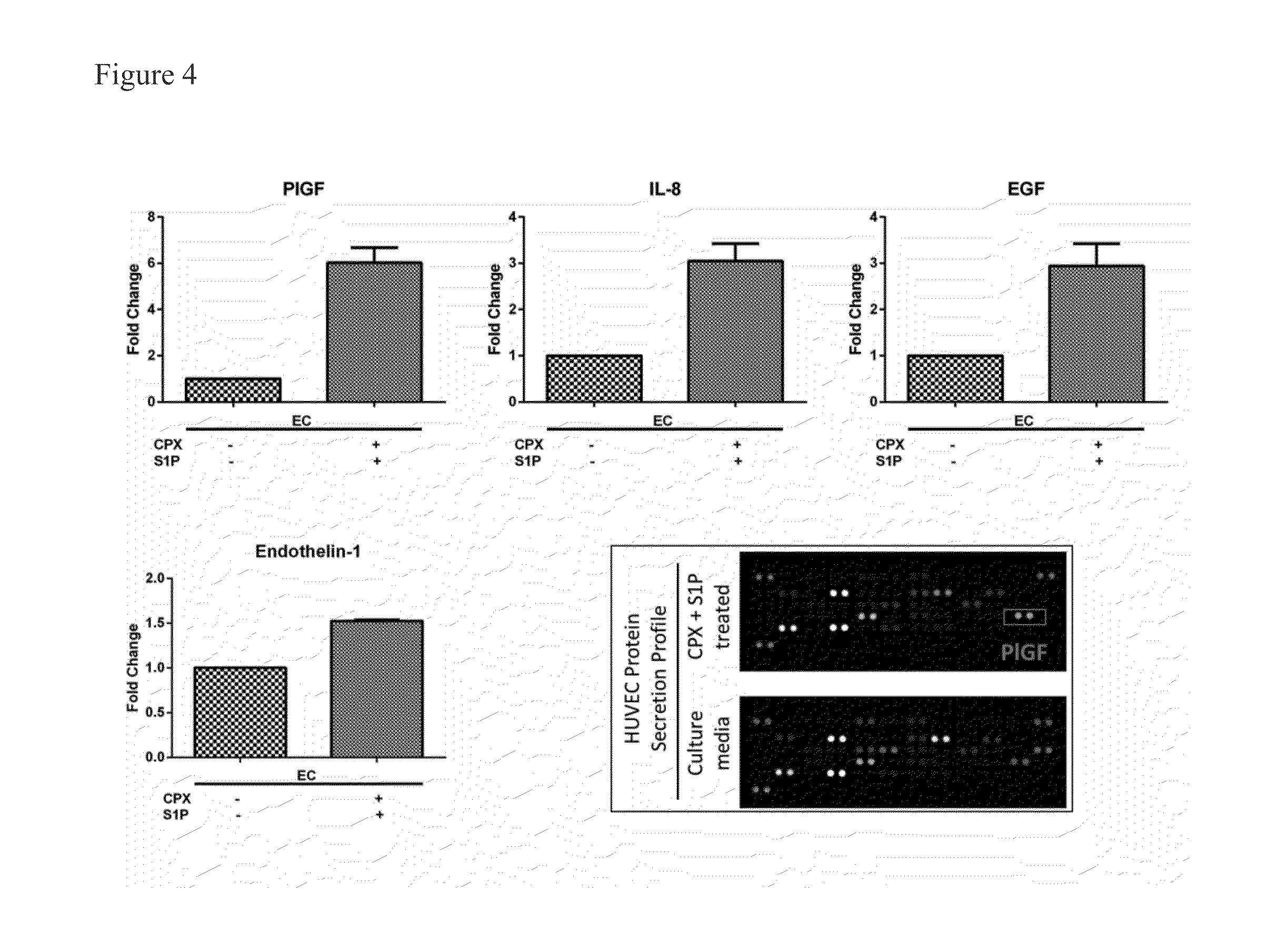
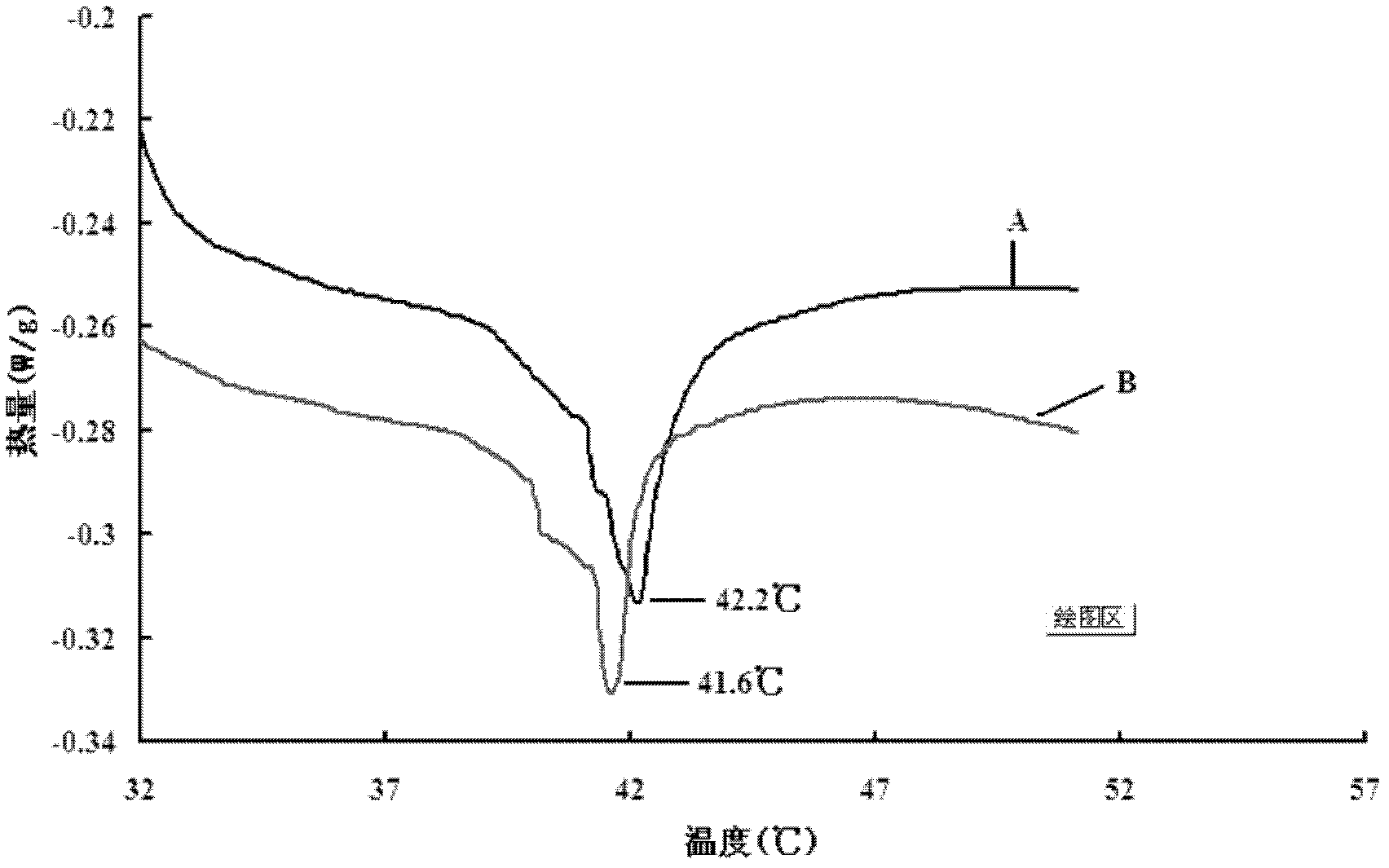
ActiveUS20130197038A1Good effectBiocideMicrobiological testing/measurementVascularizesNeovascularization
The invention is directed to a method of inducing angiogenesis at a site in an individual in need thereof comprising administering locally to the site an effective amount of one or more agents that induce hypoxia induced factor 1α (HIF-1α). In another aspect, the invention is directed to a method of inducing angiogenesis at a site in an individual in need thereof comprising administering locally to the site an effective amount of one or more agents that induce hypoxia induced factor 1α (HIF-1α) and one or more lysophospholipids. In addition, the invention is directed to methods of generating prevascularized tissue, methods of generating a vascular network in a device and compositions thereof.
Owner:MASSACHUSETTS INST OF TECH +1
Method for producing phospholipid
ActiveCN102471788AFatty substance recovery/refiningFatty acid esterificationPhospholipase A2Glycerol
Provided is a method for producing a phospholipid at a low cost by reusing phospholipase A2 in a method for producing the phospholipid whereby an arbitrary fatty acid is bonded to the 2-position of a phospholipid using an esterification reaction catalyzed by phospholipase A2 in glycerol. The method for producing a phospholipid is characterized by comprising conducting an esterification reaction catalyzed by phospholipase A2 between a lysophospholipid and an acyl donor in glycerol to form a phospholipid, adding a solvent which is immiscible with glycerol to form a glycerol layer and a solvent layer, extracting said phospholipid into said solvent layer, allowing phospholipase A2 to migrate into said glycerol layer, and, after separating the glycerol layer and distilling off the solvent remaining therein, further adding to the residual glycerol solution the lysophospholipid and the acyl donor to thereby conduct the esterification reaction again with the use of phospholipase A2 remaining in said glycerol solution.
Owner:KANEKA CORP +1
Thermosensitive liposome and its application
InactiveCN102614126AIncreased sensitivityHemolytic side effects are reducedPharmaceutical non-active ingredientsAntineoplastic agentsThermosensitive liposomesActive agent
The invention relates to a thermosensitive liposome, which contains at least one phosphatidylcholine, at least one lysophospholipid, and at least one unsaturated phospholipid. The phase temperature of the liposome is 39.0DEG C-45.0DEG C. The liposome further contains a material having a long circulating characteristic and a fat-soluble active agent. Specifically, the phosphatidylcholine, the lysophospholipid, the unsaturated phospholipid, and the material having a long cycle characteristic are in a weight ratio of 69-94:1-6:1-10:4-15. And all the phospholipids and the fat-soluble active agent are in a weight ratio of 30-120:1-10. The invention also relates to a pharmaceutical composition containing the thermosensitive liposome. The invention additionally relates to application of the thermosensitive liposome and the pharmaceutical composition in preparing drugs treating tumors.
Owner:INST OF PHARMACOLOGY & TOXICOLOGY ACAD OF MILITARY MEDICAL SCI P L A
Method for producing phospholipid
Provided is a method for producing a phospholipid at low cost by reusing phospholipase A2 in a method for producing the phospholipid whereby an arbitrary fatty acid is bonded to the 2-position of a phospholipid using an esterification reaction catalyzed by phospholipase A2 in glycerol. The method for producing a phospholipid is characterized by comprising conducting an esterification reaction catalyzed by phospholipase A2 between a lysophospholipid and an acyl donor in glycerol to from a phospholipid, adding a solvent immiscible with glycerol to form a glycerol layer and a solvent layer, extracting said phospholipid into said solvent layer, allowing phospholipase A2 to migrate into said glycerol layer, and, after separating the glycerol layer and distilling off the solvent remaining therein, further adding to the residual glycerol solution the lysophospholipid and the acyl donor to thereby conduct the esterification reaction again with use of phospholipase A2 remaining in said glycerol solution.
Owner:KANEKA CORP +1
Composition and method for controlling insect pests and diseases on plants
InactiveUS20020010157A1Effective pest controlEffective controlBiocideDead animal preservationMonoglycerideInsect pest
A composition and method for controlling diseases and pests on plants is provided. Specifically, the composition provides that polar glycerides selected from the group comprising monoglycerides, diglycerides, phospholipids, lysophospholipids, glycolipids, lysoglycolipids, or their derivatives control certain diseases or insect pests on plants.
Owner:DUAN YOUSHENG +3
Process for preparing high purity soy phoshatidylcholine without lysophosphatide
InactiveCN100500676CEasy to manufactureWide range of usesPhosphatide foodstuff compositionsSolventSoybean Phospholipids
The invention discloses a high-purity soya bean phosphatidyl choline without lysophosphatide preparing method in the phosphatide technique domain, which comprises the following steps: using soybean podwer phosphatide for raw material; extracting by dissolvant; freezing raffinate; treating liquor by dual column chromatogram; eluting for treating column 1; dislodging phosphatidyl ethanolamine; eluting and treating column2; dislodging lysophosphatide, wherein eluent of dissolvant,column 1 and column 2 for extracting is common single solvent; the production has no lysophosphatide; the bilineurine content of soya bean lecithin is more than 90%.
Owner:JIANGNAN UNIV
Novel lysophospholipid acyltransferase
ActiveUS20120115231A1Improvement in ability to produce fatty acidImprove productivityFatty oils/acids recovery from wasteFungiNucleotideNucleotide sequencing
The present invention provides novel lysophospholipid acyltransferases. The object of the present invention is attained by the nucleotide sequences of SEQ ID NOs: 1 and 6 and the amino acid sequences of SEQ ID NOs: 2 and 7 of the present invention.
Owner:SUNTORY HLDG LTD
Method For The Production Of Polyunsaturated Fatty Acids
The present invention relates to a process for producing polyunsaturated fatty acids in an organism by introducing nucleic acids into the organism which code for polypeptides having acyl-CoA:lysophospholipid a cyltransferase activity. Advantageously, these nucleic acid sequences may, if appropriate together with further nucleic acid sequences coding for biosynthesis polypeptides of the fatty acid or lipid metabolism, be expressed in the transgenic organism. The invention furthermore relates to the nucleic acid sequences, to nucleic acid constructs comprising the nucleic acid sequences of the invention, to vectors comprising the nucleic acid sequences and / or the nucleic acid constructs and to transgenic organisms comprising the abovementioned nucleic acid sequences, nucleic acid constructs and / or vectors. A further part of the invention relates to oils, lipids and / or fatty acids produced by the process of the invention and to their use.
Owner:BASF PLANT SCI GMBH
Method for detecting cancer associated with elevated concentrations of lysophospholipids
InactiveUS20020150955A1Increases specificity and sensitivityImproved prognosisBiological testingBody fluidLysophospholipids
The present invention is methods for detecting the presence of cancer in a subject by determining the concentrations of lysophospholipids in a sample of bodily fluid taken from a test subject and comparing these concentrations to concentrations present in samples taken from normal subjects without cancer. The methods may be used for diagnosis and prognosis of cancer in a subject and to monitor the results of therapy of over time.
Owner:MILLS GORDON B +2
Preparation method of high-purity glycerophosphate diester
The invention belongs to the technical field of gene engineering of enzymes, and discloses a preparation method of high-purity glycerophosphodiesters, which comprises the following steps: hydrolyzing lysophosphatide in a water phase system by taking lysophosphatide as a raw material and pfGDPD treated by a metal ion chelating agent as a catalyst to obtain a glycerophosphodiester product; the amino acid sequence of the pfGDPD is shown as SEQ ID No: 1. Starting from lysophospholipid, high-purity glycerophosphodiesters (such as GPC, GPE, GPI and the like) can be prepared through one step of hydrolysis by a single enzyme method, and the conversion rate is close to 100%. The invention provides a new thought and method for the production of glycerophosphate diester.
Owner:SOUTH CHINA UNIV OF TECH
EPA/DHA type lysophospholipid composition and preparation method thereof
The invention discloses an EPA / DHA type lysophospholipid composition and a preparation method thereof, the composition comprises the following components: less than or equal to 25% of phospholipid PC,more than or equal to 65% of lysophospholipid LPC, and less than or equal to 10% of glycerocholine phosphate GPC, and the content of EPA / DHA in the lysophospholipid LPC is greater than 60%. The method comprises the following steps: taking phospholipid in the krill oil as a raw material, taking phospholipase A1 as a catalyst to catalyze an alcoholysis reaction of the phospholipid, ending the reaction when 80% of phospholipid substances in the krill oil are subjected to the alcoholysis reaction, adding cold acetone, performing standing or centrifuging, and taking a lower-layer phospholipid mixture; taking a phospholipid mixture obtained in the step 1 as a raw material, taking immobilized partial glyceride lipase as a catalyst, catalyzing the reaction of a byproduct GPC in the phospholipid mixture obtained in the step 1 with EPA / DHA and ester derivatives thereof, after the reaction is finished, adding cold acetone, performing standing or centrifuging, and taking the lower-layer phospholipid mixture, thereby obtaining the composition taking EPA / DHA lysophospholipid as a main component. The composition provided by the invention is rich in EPA / DHA type lysophospholipid and has an important health-care function.
Owner:大渔华创(广州)海洋生物科技有限公司
Elemene injection and preparation method thereof
InactiveCN101708161AResolve hemolysisResolve vascular irritationOrganic active ingredientsPharmaceutical non-active ingredientsPhosphateIrritation
The invention discloses an elemene injection. One liter of the injection consists of the following raw materials: 5 to 20 grams of elemene, 15 to 60 grams of soybean lecithin for injection, 5 to 99.9 grams of oil for injection, 12 to 30 grams of glycerol for injection, and the balance of phosphate buffer solution. The invention also discloses a method for preparing the elemene injection. By controlling the content of lysophosphatide in an auxiliary material, namely soybean lecithin, and key preparation process conditions, an anti-cancer medicament, namely the elemene injection, is prepared, which has no hemoclasis, no vascular irritation and no risk of the survival of microorganisms after the sterilization, is suitable for intravenous injection or arterial intervention, has a dosage form of medicament-loaded emulsions, and is safe and effective.
Owner:JINGANG PHARMA DALIAN
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com