Vaccine cryoprotectant without composition of gelatin and human albumin
A technology for freeze-drying protective agent and human blood, which can be applied to medical preparations without active ingredients, medical preparations containing active ingredients, and antibody medical ingredients, etc. Harmful, low cost, good safety effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
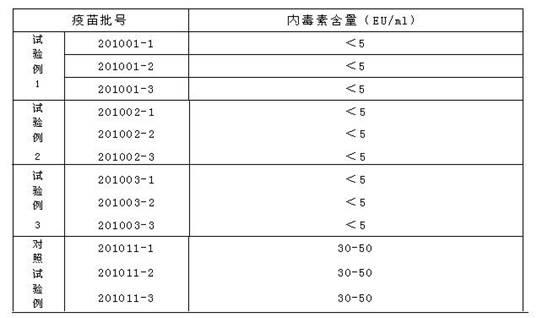
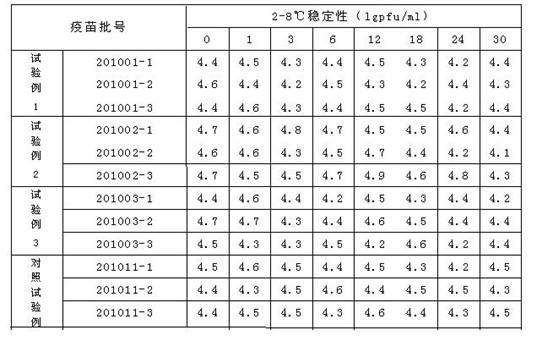
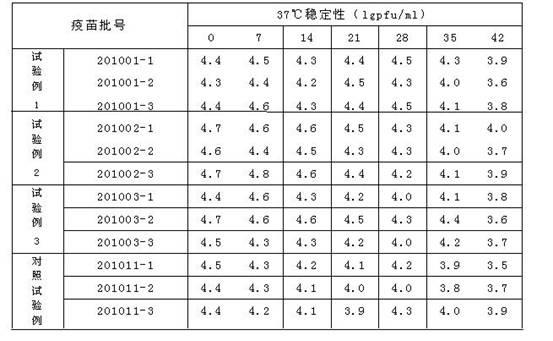
[0017] Weigh the following substances (g / l): dextran (40) 50, sucrose 50, lactose 20, mannitol 10, glycine 10, arginine 1.85, sodium glutamate 9.2, urea 4.6, 199 comprehensive medium 2, Fully dissolve the above-mentioned substances in an appropriate amount of sterilized water for injection, then constant volume, measure the osmotic pressure within the range of 300-450mosm, adjust the pH with 1N hydrochloric acid to within the range of 7.2-7.8, and finally sterilize and filter to obtain the obtained product.
Embodiment 2
[0019] Weigh the following substances (g / l): dextran (40) 20, sucrose 30, lactose 10, mannitol 5, glycine 5, arginine 1.85, sodium glutamate 9.2, urea 4.6, 199 comprehensive medium 2, Fully dissolve the above-mentioned substances in an appropriate amount of sterilized water for injection, then constant volume, measure the osmotic pressure within the range of 300-450mosm, adjust the pH with 1N hydrochloric acid to within the range of 7.2-7.8, and finally sterilize and filter to obtain the obtained product.
Embodiment 3
[0021] Weigh the following substances (g / l): dextran (40) 60, sucrose 60, lactose 25, mannitol 15, glycine 15, arginine 1.85, sodium glutamate 9.2, urea 4.6, 199 comprehensive medium 2, Fully dissolve the above-mentioned substances in an appropriate amount of sterilized water for injection, then constant volume, measure the osmotic pressure within the range of 300-450mosm, adjust the pH with 1N hydrochloric acid to within the range of 7.2-7.8, and finally sterilize and filter to obtain the obtained product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com