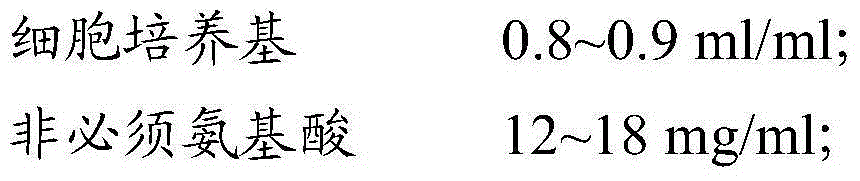Cell cryo-preserved liquid, application, and immune cell cryo-preservation method
A technology of immune cells and cryopreservation methods, which can be used in applications, preservation of human or animal bodies, animal husbandry, etc., and can solve problems such as danger, immune rejection, and infectious diseases.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] 1.1 Take 20ml of peripheral blood or umbilical cord blood, add an equal volume of normal saline to dilute, add one-third of the diluent’s volume of Ficoll separation solution, centrifuge at 700g for 20-30min, reduce the lifting speed to zero, and absorb the middle buffy coat.
[0057] 1.2 Wash the buffy coat obtained in 1.1 twice with normal saline. According to the counting results, the inoculation density is 1×10 6 cell / ml, inoculate into RPMI1640 medium containing 10% FBS, add 1000UI / ml interferon-γ, add 300UI / ml interleukin-2 and 300UI / ml CD3 monoclonal antibody after 24h; add fluid on the 4th day, fluid density 5~10×10 5 cell / ml, and add 300 UI / ml of interleukin 2 according to the volume of rehydration; follow-up rehydration once every 3 days, and the density after rehydration is maintained at 1-1.5×10 6 cell / ml, and add 300UI / ml of interleukin 2 according to the volume of rehydration; culture for 12 to 14 days to obtain CIK cells.
[0058] 1.3 Pour out the CIK c...
Embodiment 2
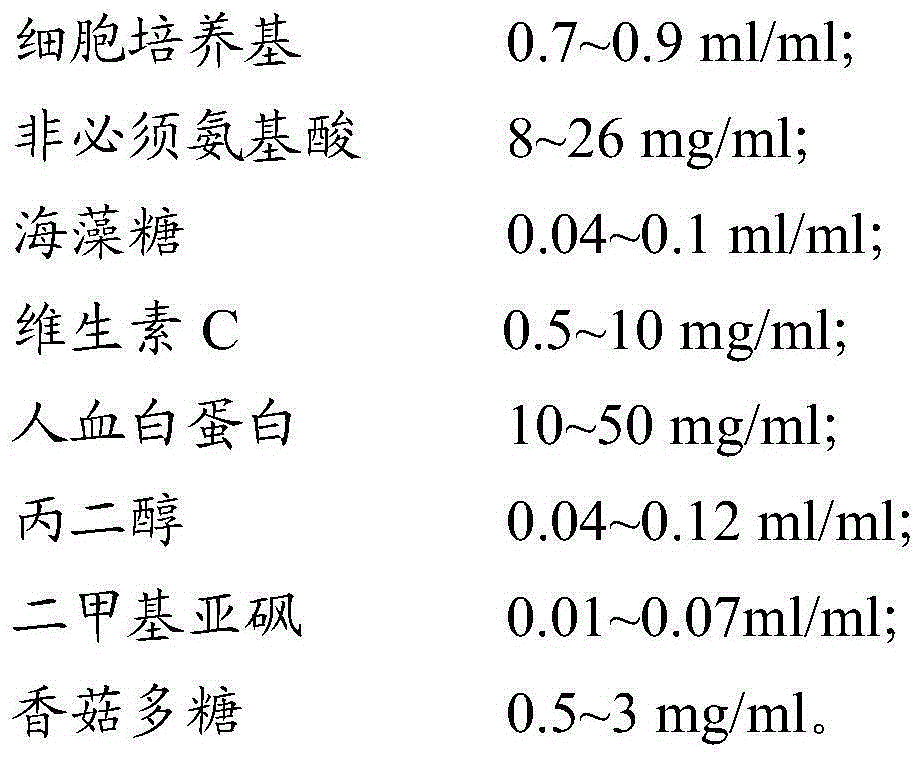
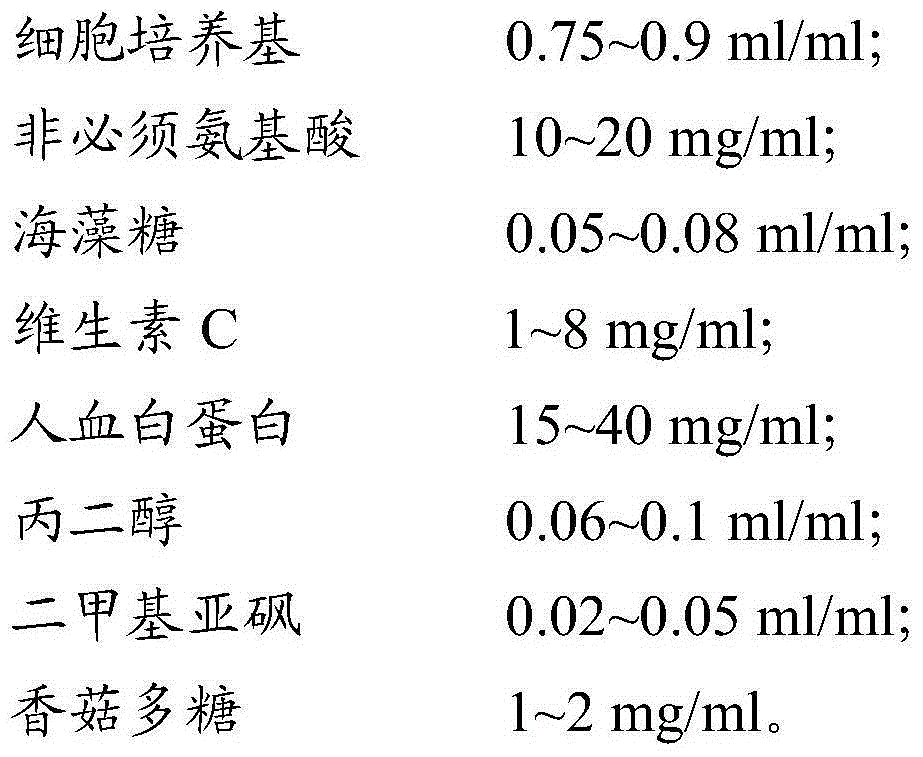
[0064] 2.1 Pour out the CIK cells obtained in Example 1 from the culture flask, centrifuge at 400g for 5min, discard the supernatant and wash 2 times with PBS. After discarding the PBS, add the cell freezing solution (see the formula for the cell freezing solution) Table 2) Resuspended cells at a cell density of 3×10 7 cell / ml; take a part of the cell suspension for trypan blue staining to calculate the activity, and at the same time detect the surface markers CD3 and CD56 of CIK by flow cytometry; add the remaining cell suspension to cryopreservation tubes, divide each tube into 10 Day, 30 days, and 60 days in three groups, with 3 tubes of cells in each group, a total of 9 tubes of cells; the cryopreservation tubes were stored in a -80°C refrigerator, and transferred to liquid nitrogen for storage after 24 hours.
[0065] 2.2 Recovery of immune cells: Take out the immune cells stored in liquid nitrogen for 10 days, 30 days, and 60 days, and thaw them in a water bath at 37°C. ...
Embodiment 3
[0069] 3.1 Pour out the CIK cells obtained in Example 1 from the culture flask, centrifuge at 400g for 5min, discard the supernatant and wash with PBS twice, after discarding the PBS, add the cell freezing solution (see the formula for the cell freezing solution) Table 3) Resuspended cells at a cell density of 3×10 7 cell / ml; take a part of the cell suspension for trypan blue staining to calculate the activity, and at the same time detect the surface markers CD3 and CD56 of CIK by flow cytometry; add the remaining cell suspension to cryopreservation tubes, divide each tube into 10 Day, 30 days, and 60 days in three groups, with 3 tubes of cells in each group, a total of 9 tubes of cells; the cryopreservation tubes were stored in a -80°C refrigerator, and transferred to liquid nitrogen for storage after 24 hours.
[0070] 3.2 Recovery of immune cells: Take out the immune cells stored in liquid nitrogen for 10 days, 30 days, and 60 days, and thaw them in a water bath at 37°C. Th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com