Test strip for semi-quantitative detection of microalbuminuria
A technology for urine microalbumin and semi-quantitative detection, which is applied in biological testing, material testing, etc., to achieve the effects of simple operation, convenient use and wide detection range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
[0060] Experimental example 1. the preparation method of test strip of the present invention
[0061] Biologically active raw materials: anti-human albumin monoclonal antibody, rabbit IgG, anti-rabbit IgG polyclonal antibody and human albumin are all imported from Canada Artron BioResearch Inc.
[0062] Reagents: bovine serum albumin (BSA) and casein were purchased from Sigma.
[0063] Nitrocellulose membrane: Sartorius, Germany
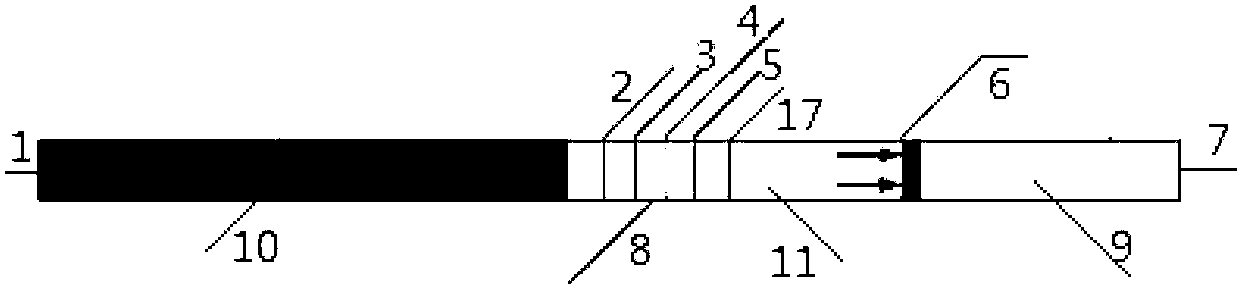
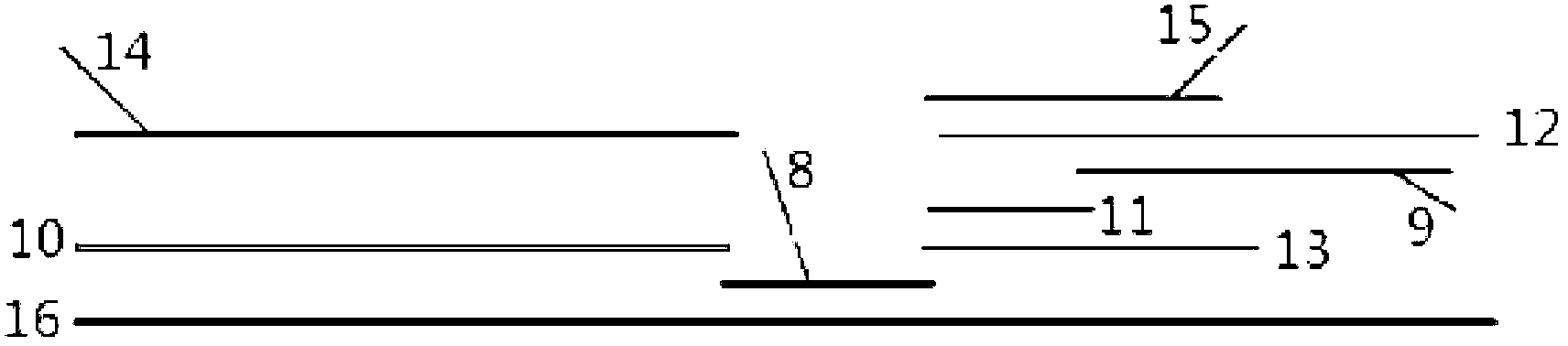
[0064] The test strip preparation procedure is as follows
[0065] Step 1. Prepare coating solution
[0066] Dilute anti-rabbit IgG polyclonal antibody and human albumin with 20mM PBS, prepare anti-rabbit IgG polyclonal antibody to 2mg / ml, and human albumin to 0.3mg / ml, respectively for the C and T lines on the nitrocellulose membrane Covered.
[0067] Step 2. Coating of reagents
[0068] Anti-rabbit IgG polyclonal antibody and human albumin coated on nitrocellulose membrane:
[0069] Coat human albumin and anti-rabbit IgG polyclonal antibody o...
Embodiment 2
[0088] Embodiment 2. Using method and methodological comparative experiment
[0089] How to use this product:
[0090] Take the urine to be tested and drop it on the sample fiber pad of the horizontal test strip, or immerse the part of the mucus end of the test strip below the MAX line in the urine for about 5 seconds, take it out and place it horizontally, and interpret the result within 5 minutes.
[0091] Comparison 1: Product Comparison
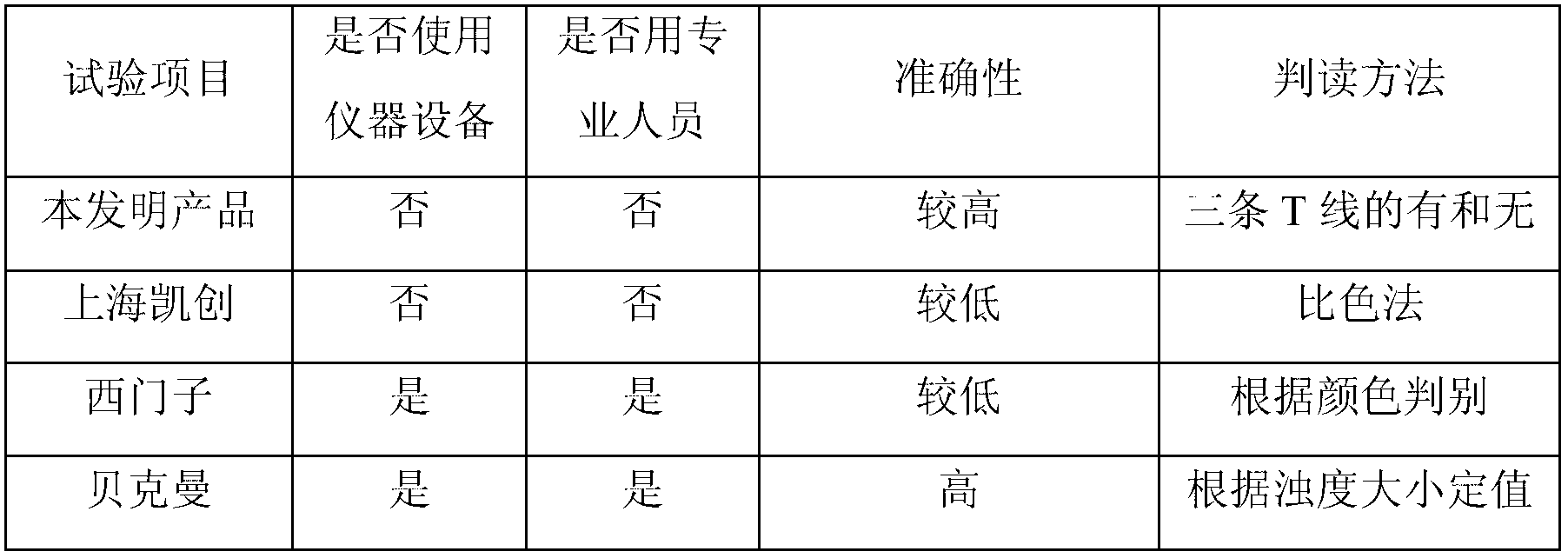
[0092] The product of Example 1 of the present invention is fast, accurate, specific, and convenient compared with the prior art products for detecting urine microalbumin, and there is no need for necessary instrument cleaning and maintenance before and after detecting urine microalbumin work, and does not require professionals in the field to operate.
[0093] Control product: Roche Diagnostic Products (Shanghai) Co., Ltd. Microalbumin Assay Kit (Immunoturbidimetric Method) State Food and Drug Administration (Jin) Zi 2011 No. 2402465 ...
Embodiment 3
[0106] Compared with the invention patent with the application number of 200810305085.9 in Example 1 of the present invention, a semi-quantitative detection of urine microalbumin colloidal selenium test paper can not only be judged whether it is a normal level, but also can determine the concentration exceeding the normal level. Wide range.
[0107] The comparative data are shown in Table 3:
[0108]
[0109]
[0110] Result analysis: the colloidal gold test paper can semi-quantitatively detect the specific values of 20mg / L, 50mg / L, 100mg / L, and >100mg / L, while the colloidal selenium test paper can only judge whether it is a normal level (<20mg / L) , or an abnormal level (≥20mg / L), it should actually be listed as a qualitative product, and it cannot be called a semi-quantitative test strip.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com