Use of cannabinoids and terpenes for treatment of organophosphate and carbamate toxicity
a terpene and cannabinoid technology, applied in the direction of biocide, drug composition, peptide/protein ingredients, etc., can solve the problems of increasing the difficulty of cholinergic crisis treatment, many casualties alarming the international community about the risks to the stability of the region, and around 300,000 deaths a year. , to achieve the effect of preventing the breakdown of the neurotransmitter ach and increasing the level and duration of action of this neurotransmitter
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0145]OP toxicity was determined using DFP toxicity. Male and female albino rats (total 4 rats) were singly dosed subcutaneously with different amounts of the DFP. Severity of salivation, lacrimation, urination and defecation (SLUD) signs were measured at dose levels of 0.63, 1.88, 1.25, and 2.5 DFP mg / kg. Dose escalation is used if the previous doses used do not, induce sufficient responses to test efficacy of the test article such as abnormal pupil response. SLUD, arousal, abnormal motor movement, gait and posture.
example 2
[0146]The same protocol as in Example 1 is conducted in a primate to measure the OP DFP toxicity by monitoring the same parameters to find a dose level that would provide clear and measurable evidence of DFP toxicity in a primate.
example 3
[0147]In order to test the onset of cannabinoid and terpene activities in rats, a combination of THC, CBD, and terpenes were administered to the animals. In accordance with an IRB approved protocol, a first group of rats were administered a terpene blend for 20 seconds of sniffing of the blend. After sniffing of the terpene blend, different combinations of cannabinoids by themselves or in cannabinoid / terpene blends were administered by oral gavage.
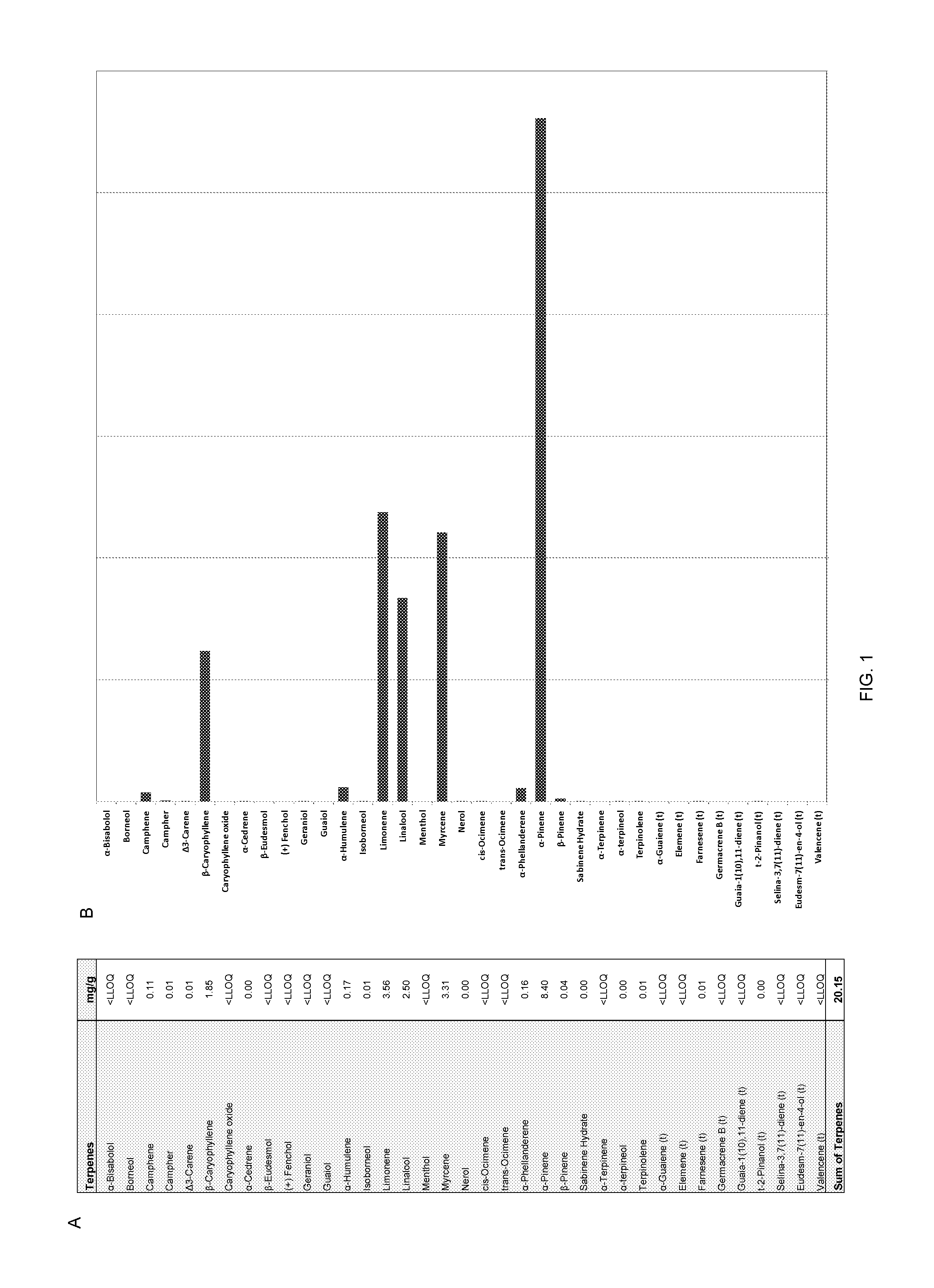
[0148]At least one of the blend combinations was composed of D-limonene:alpha pinene:myrcene, linalool:beta caryophylene in volumetric ratios of about 4:4:3:3:1 respectively to make approximately a 30 mg / kg dose. The same method may be used to explore other combinations within the scope of the ratios described herein above, such as for example, a terpene blend containing limonene, alpha pinene, myrcene, linalool and beta carylophylene preferably in weight ratios of about 4:7:3:3:2 as described in FIG. 1.
[0149]In another experiment the anim...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight ratios | aaaaa | aaaaa |
| surface area | aaaaa | aaaaa |
| skin surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com