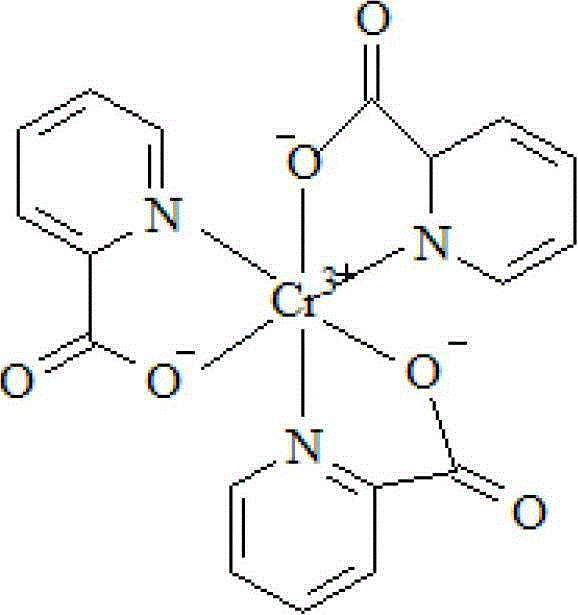
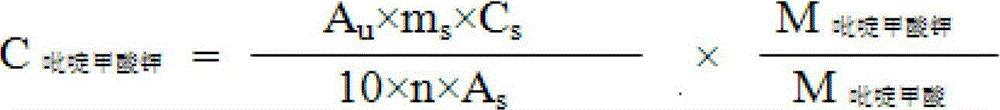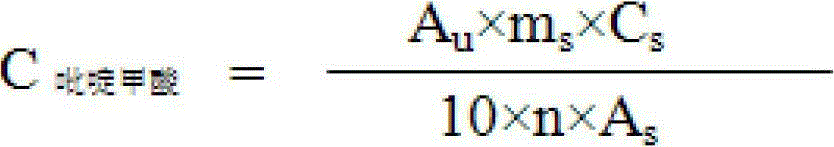Synthesis method of chromium 2-pyridylformate
A technology of chromium picolinate and a synthesis method, which is applied in the field of synthesizing chromium 2-picolinate, can solve the problems of dangerous reaction, long reaction time, difficult reaction control, etc., achieves guaranteed yield and quality, saves raw material cost, and saves reaction raw materials Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0078] (1) Oxidation reaction
[0079] Add 20mL of 2-picoline (0.2mol, density of 0.943g / mL, purity of 99.5%), phase transfer catalyst tetrabutylammonium bromide 0.19g (0.0006mol) into a 500mL reactor, and add 200mL of laboratory water , heating, stirring, adding potassium permanganate in 6 times, 15.8g each time, that is, the total amount of potassium permanganate added is 94.8g (0.5mol), after adding the last batch of potassium permanganate, react for 30min Afterwards, the reaction system changed from purple to black, that is, the potassium permanganate was completely reacted, kept at 85°C, and continued to react for 2 hours. The obtained reaction solution was filtered while it was hot, washed and filtered three times with 45mL hot water (15mL each time). cake, filter with suction, remove the black filter cake, combine the filtrate and washing liquid to obtain a mixture, and cool to 15°C. Using a Shimadzu A1800 ultraviolet spectrophotometer to analyze according to the ultra...
Embodiment 2
[0083] (1) Oxidation reaction
[0084] Add 40mL of 2-methylpyridine (0.4mol, density of 0.943g / mL, purity of 99.5%), phase transfer catalyst tetrabutylammonium chloride 11.1g (0.04mol) into a 1L reactor, and add 400mL of laboratory water , heating, stirring, adding potassium permanganate in 4 times, 31.6g each time, that is, the total amount of potassium permanganate added is 126.4g (0.8mol), after adding the last batch of potassium permanganate, react for 30min Afterwards, the reaction system changed from purple to black, that is, the potassium permanganate was completely reacted, kept at 95°C, and continued to react for 1 hour. The obtained reaction solution was filtered while it was hot, washed and filtered three times with 90mL hot water (30mL each time). cake, filter with suction, remove the black filter cake, combine the filtrate and washing liquid to obtain a mixed solution, cool to 20°C, and use a Shimadzu A1800 UV spectrophotometer to analyze according to the UV spect...
Embodiment 3
[0088] (1) Oxidation reaction
[0089]Add 60mL of 2-methylpyridine (0.6mol, density 0.943g / mL, purity 99.5%) and 15.0g (0.04mol) of phase transfer catalyst methyl triphenyl bromide into the 1L reactor. Heat 600mL of water, stir, add potassium permanganate in 5 times, 47.4g each time, that is, the total amount of potassium permanganate added is 237.0g (1.5mol), when the last batch of potassium permanganate is added, After 30 minutes of reaction, the reaction system changed from purple to black, that is, the potassium permanganate was completely reacted, kept at 85°C, and continued to react for 1 hour. The obtained reaction solution was filtered while it was hot, and divided into four times with 100mL hot water (25mL each time) ) to wash the filter cake, filter with suction, remove the black filter cake, combine the filtrate and washing liquid to obtain a mixed solution, and cool to 25°C. Using a Shimadzu A1800 ultraviolet spectrophotometer to analyze according to the ultraviol...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com