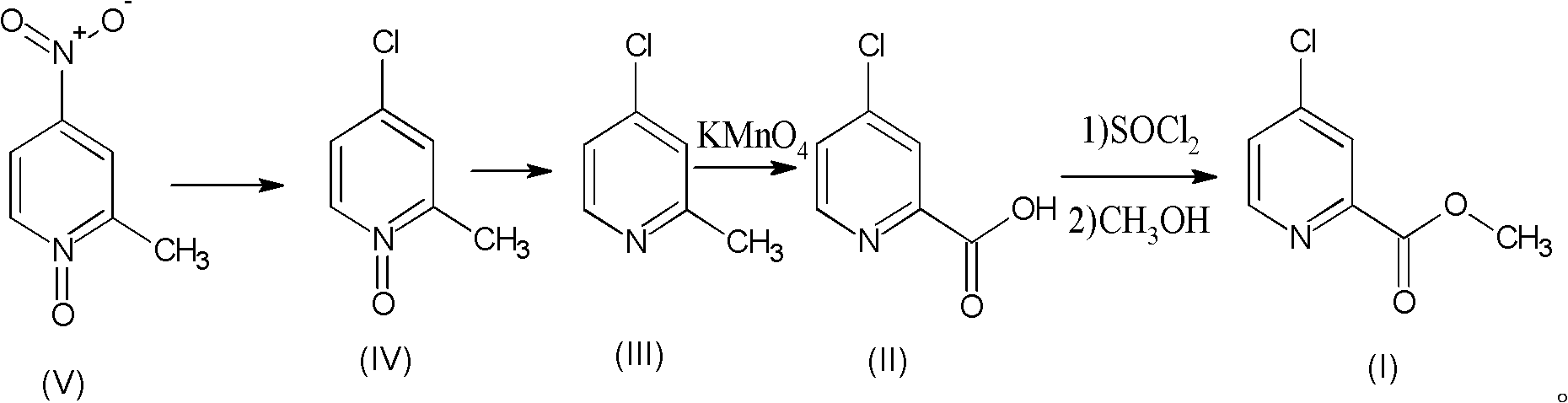
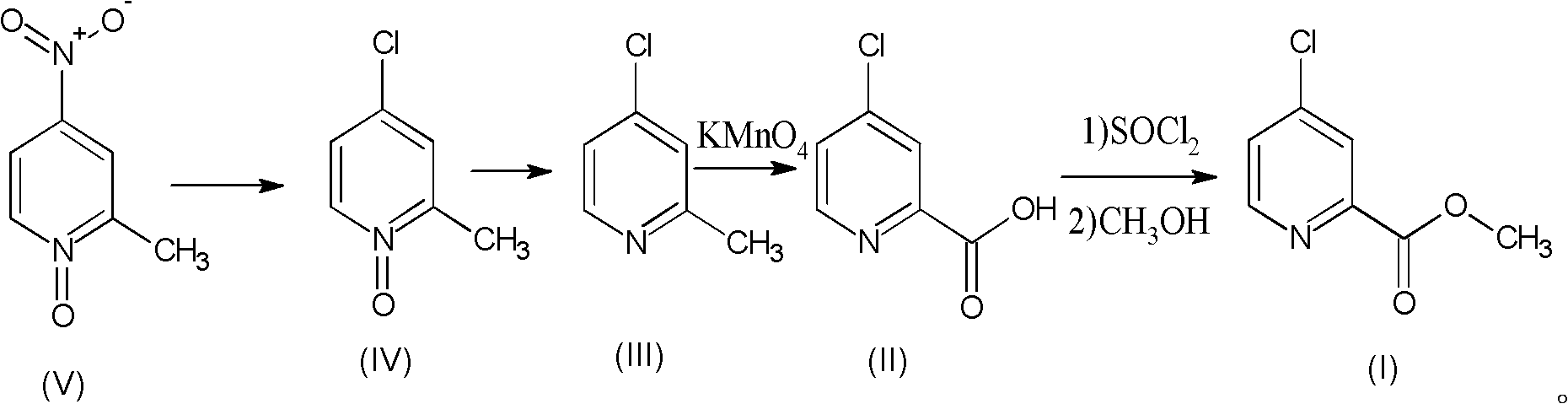
Preparation process of high-purity 4-chloro-2-pyridinecarboxylate hydrochloride
A technology for the preparation of methyl picolinate and a preparation process, which is applied in the field of preparation of intermediates of medicines and pesticides, can solve the problems of low product purity and inconvenient source of 4-chloropyridine raw materials, and achieve high product yield, easy industrial production, and raw materials Source Rich Effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Compound V (2-methyl-4-nitropyridine-N-oxide), commercially available, the following examples are the same.
[0035] (1) Preparation of 4-chloro-2-methyl-pyridine-N-oxide (compound IV)
[0036] 15.4g (0.10mol) of 2-methyl-4-nitropyridine-N-oxide (compound V) (and 180ml of concentrated hydrochloric acid were added to the autoclave, heated to 180°C, and reacted for 24 hours. After the end, use NaOH Adjust the pH to 6-7, extract with chloroform, and distill under reduced pressure to obtain 4-chloro-2-methylpyridine-N-oxide (compound IV) to obtain 11.7g 4-chloro-2-methyl-pyridine-N- Oxides, HPLC (high performance liquid chromatography, High Performance Liquid Chromatography, referred to as HPLC) measured purity 98.7%, reduced pure 11.55g, yield 80.4%.
[0037] (2) Preparation of 4-chloro-2-picoline (compound III)
[0038] Mass / volume ratio compound VI: organic solvent = 1: 6.96
[0039] Molar ratio compound VI: phosphorus trichloride=1:2.13
[0040] Add 11.5g (0.08mol) ...
Embodiment 2
[0050] (1) Preparation of 4-chloro-2-methyl-pyridine-N-oxide (compound IV)
[0051] 15.4g (0.10mol) of 2-methyl-4-nitropyridine-N-oxide (compound V) and 180ml of concentrated hydrochloric acid were added into the autoclave, heated to 250°C, and reacted for 8 hours. After the end, adjust the pH to 6-7 with NaOH, extract with chloroform, and distill under reduced pressure to obtain 4-chloro-2-methylpyridine-N-oxide, and obtain 5.8g of 4-chloro-2-methyl-pyridine-N- Oxide (HPLC purity 97.6%), pure 5.66g, yield 39.4%.
[0052] (2) Preparation of 4-chloro-2-picoline (compound III)
[0053] Mass / volume ratio: compound VI: organic solvent = 1: 3.74
[0054] Molar ratio compound VI: phosphorus trichloride=1:1.5
[0055] Add 11.5g (0.08mol) 4-chloro-2-picoline-N-oxide into the three-necked flask, dissolve it in 43ml of dichloromethane, cool down to -10°C, add 10.5ml (0.12 mol) phosphorus trichloride. The temperature was raised to 40° C. for 6 hours. Cool down to room temperature, ...
Embodiment 3
[0065] (1) Preparation of 4-chloro-2-methyl-pyridine-N-oxide (compound IV)
[0066] 15.4g (0.10mol) of 2-methyl-4-nitropyridine-N-oxide (compound V) and 180ml of concentrated hydrochloric acid were added into the autoclave, heated to 120°C, and reacted for 30 hours. After the end, adjust the pH to 6-7 with NaOH, extract with chloroform, and distill under reduced pressure to obtain 4-chloro-2-methylpyridine-N-oxide (compound IV) to obtain 5.0 g of 4-chloro-2-methyl -Pyridine-N-oxide (HPLC purity 99.1%), pure 4.96g, yield 34.5%.
[0067] (2) Preparation of 4-chloro-2-picoline (compound III)
[0068] Mass / volume ratio: compound VI: organic solvent = 1: 11.22
[0069] Molar ratio compound VI: phosphorus trichloride=1:3
[0070] Add 11.5g (0.08mol) 4-chloro-2-methylpyridine-N-oxide (compound IV) into the three-necked flask, dissolve it in 129ml carbon tetrachloride, cool down to 10°C, add dropwise within 1 hour 21.0ml (0.24mol) phosphorus trichloride. Raise the temperature to ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com