Method for preparing 2-chloro-6-trichloromethylpyridine
A technology of trichloromethylpyridine and picoline, which is applied in the field of industrial preparation of 2-chloro-6-trichloromethylpyridine, can solve problems such as low productivity, increased production cost, and expensive raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
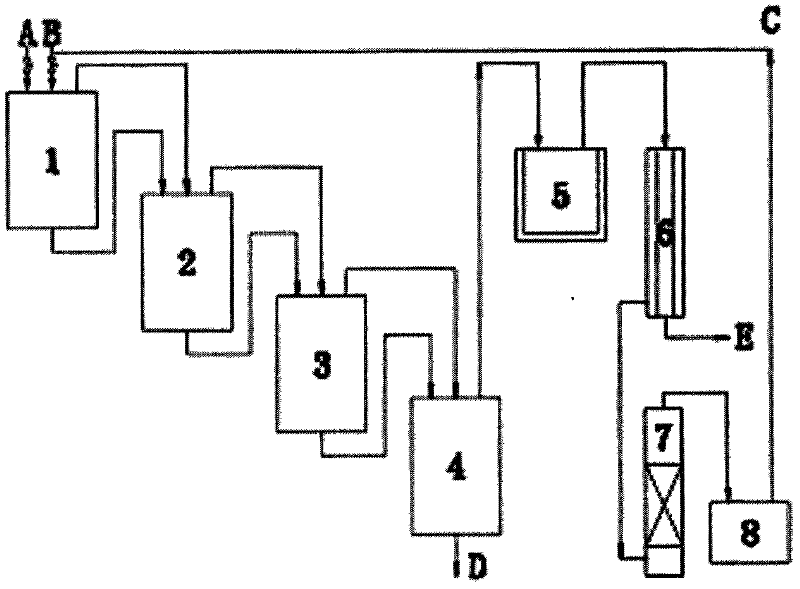
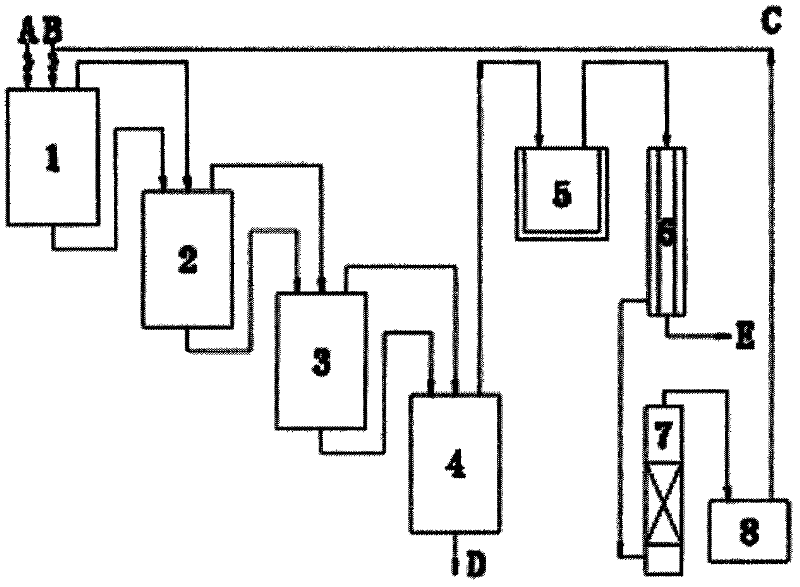
[0042] When the temperature of the primary chlorination tank 1 reaches 190-200°C, dry chlorine gas B is introduced at 180kg / h, and 2-picoline A is continuously added at 30kg / h; the chlorinated semi-finished product continuously flows into the secondary chlorination tank 2 , where 108kg / h of chlorine gas is introduced separately, and the temperature is controlled at 190-200°C; the temperature of the three-stage chlorination kettle 3 is 190-200°C, and chlorine gas is separately passed at 90kg / h; the temperature of the fourth-stage chlorination kettle 4 is 190-200°C ℃, gas chromatographic tracking, chlorine gas is fed separately at 72kg / h. The crude product D of 2-chloro-6-trichloromethylpyridine flowing out continuously has a content of 94% and a yield of 88%. The 2-chloro-6-trichloromethylpyridine product with a content of 99% was obtained through vacuum distillation.
[0043] The HCl tail gas produced by the reaction also contains excess chlorine gas. First, absorb HCl gas wi...
Embodiment 2
[0046] When the temperature of the primary chlorination tank 1 reaches 80°C, chlorine gas B is introduced at 180 kg / h, and 2-picoline A is continuously added at 18 kg / h at the same time; the chlorinated semi-finished product continuously flows into the secondary chlorination tank 2, where Chlorine gas is fed separately at 180kg / h, and the temperature is controlled at 230°C; the temperature of the third-stage chlorination kettle 3 is 80°C, and chlorine gas is passed through separately at 18g / h; h Chlorine gas is introduced separately. The crude product D of 2-chloro-6-trichloromethylpyridine flowing out continuously has a content of 88% and a yield of 85%. The 2-chloro-6-trichloromethylpyridine product with a content of 99% was obtained through vacuum distillation.
[0047] The HCl tail gas produced by the reaction also contains excess chlorine gas. First, absorb HCl gas with water to produce hydrochloric acid E. After the remaining wet chlorine gas is dried in a concentrated ...
Embodiment 3
[0050] When the temperature of the primary chlorination kettle 1 reaches 210°C, feed dry chlorine gas B at 180kg / h, and simultaneously add 2-picoline A continuously at 45kg / h; the chlorination semi-finished product continuously flows into the secondary chlorination kettle 2, and Chlorine gas is fed separately at 18kg / h, and the temperature is controlled at 80°C; the temperature of the third-stage chlorination kettle 3 is 230°C, and chlorine gas is passed through separately at 180g / h; / h Chlorine gas is introduced separately. The crude product D of 2-chloro-6-trichloromethylpyridine flowing out continuously has a content of 90% and a yield of 86%. The 2-chloro-6-trichloromethylpyridine product with a content of 99% was obtained through vacuum distillation.
[0051] The HCl tail gas produced by the reaction also contains excess chlorine gas. First, absorb HCl gas with water to produce hydrochloric acid E. After the remaining wet chlorine gas is dried through the concentrated su...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com