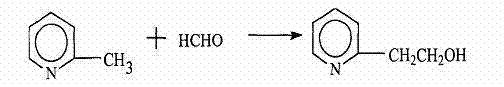Preparation method of 2-ethoxyl pyridina
A technology of hydroxyethyl pyridine and picoline, which is applied in the field of preparation of 2-hydroxyethyl pyridine, can solve the problems of high production cost, low production efficiency, low yield and the like, and achieves low production cost, high production efficiency, The effect of high product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] (1) In a 1000ml four-neck flask equipped with a thermometer, a magnetic stirrer, and a reflux condenser, add 93.13g of 2-picoline, 107.12g of lithium diisopropylamide, and 176.2ml of tetrahydrofuran, and fill the reaction system with Nitrogen protection was carried out, and the reaction was carried out under the condition of stirring and temperature of -60° C. for 1 hour to obtain a reaction liquid. (2) Add 27.60g of paraformaldehyde to the above reaction solution, stir and react for 1 hour under the reaction conditions of 10°C temperature, then add 18g of water dropwise under the above reaction conditions, and the drop time is 0.5 hours. The reaction was continued for 0.5 hour under the temperature condition of 10° C. to obtain the product liquid. (3) Add calcium sulfate to the above-mentioned product liquid, carry out dehydration treatment, filter the product liquid after removing moisture, carry out vacuum distillation on the filtrate under the conditions of temperat...
Embodiment 2
[0022] (1) In a 1000ml four-neck flask equipped with a thermometer, a magnetic stirrer, and a reflux condenser, add 93.13g of 2-picoline, 160.68g of lithium diisopropylamide, and 220.3ml of tetrahydrofuran, and fill the reaction system with Protected by nitrogen, the mixture was stirred and reacted for 2.5 hours at -30°C to obtain a reaction liquid. (2) Add 36.32g of paraformaldehyde to the above reaction solution, stir and react for 3 hours under the reaction conditions of 30°C, then add 21.6g of water dropwise under the above reaction conditions for 1 hour, after the addition is completed The reaction was continued at 40°C for 1 hour to obtain a product solution. (3) Add aluminum oxide to the above product liquid, perform water removal treatment, filter the product liquid after removing water, and carry out vacuum distillation on the filtrate at a temperature of 65°C and -0.859~-0.98MPa to remove tetrahydrofuran, That is, the target product 2-hydroxyethylpyridine was obtain...
Embodiment 3
[0024] (1) In a 1000ml four-neck flask equipped with a thermometer, a magnetic stirrer, and a reflux condenser, add 93.13g of 2-picoline, 214.24g of lithium diisopropylamide, and 440.6ml of tetrahydrofuran, and fill the reaction system with Nitrogen protection was carried out, and the reaction was carried out under the condition of stirring and -10° C. for 4 hours to obtain a reaction solution. (2) Add 44.98g of paraformaldehyde to the above reaction solution, stir and react under the reaction conditions of 60°C for 5 hours, and then under the above reaction conditions, add 27g of water dropwise for 1.5 hours. The reaction was continued at 60°C for 1.5 hours to obtain a product solution. (3) Add 4A molecular sieves to the above product liquid, perform water removal treatment, filter the product liquid after removing water, and conduct vacuum distillation on the filtrate at a temperature of 60°C and -0.859~-0.98MPa to remove tetrahydrofuran, That is, the target product 2-hydro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com