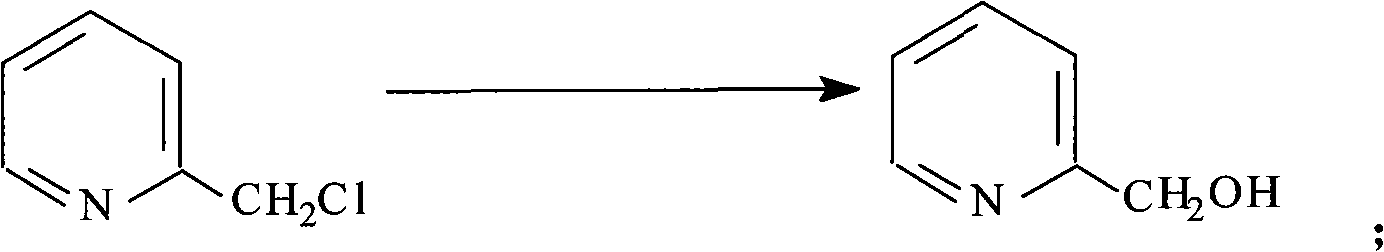
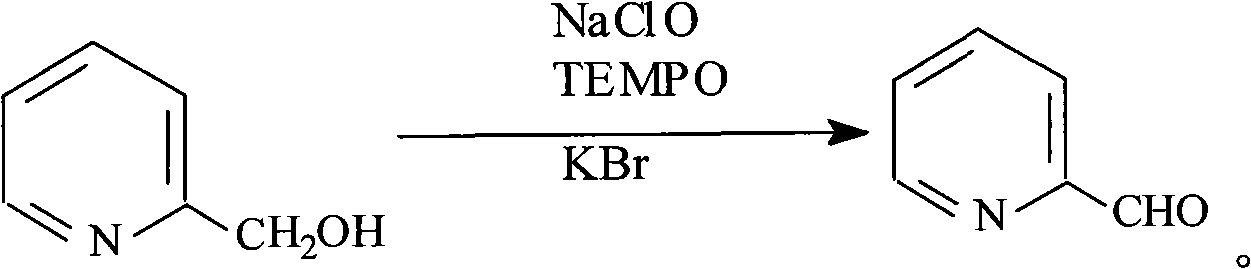Preparation method of 2-pyridine carboxaldehyde
A technology of pyridine formaldehyde and pyridine methanol, which is applied in chemical instruments and methods, physical/chemical process catalysts, organic compounds/hydrides/coordination complex catalysts, etc., can solve the problems of high production cost and high price of 2-cyanopyridine problem, to achieve the effect of low production cost, easy industrial scale production and complete reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
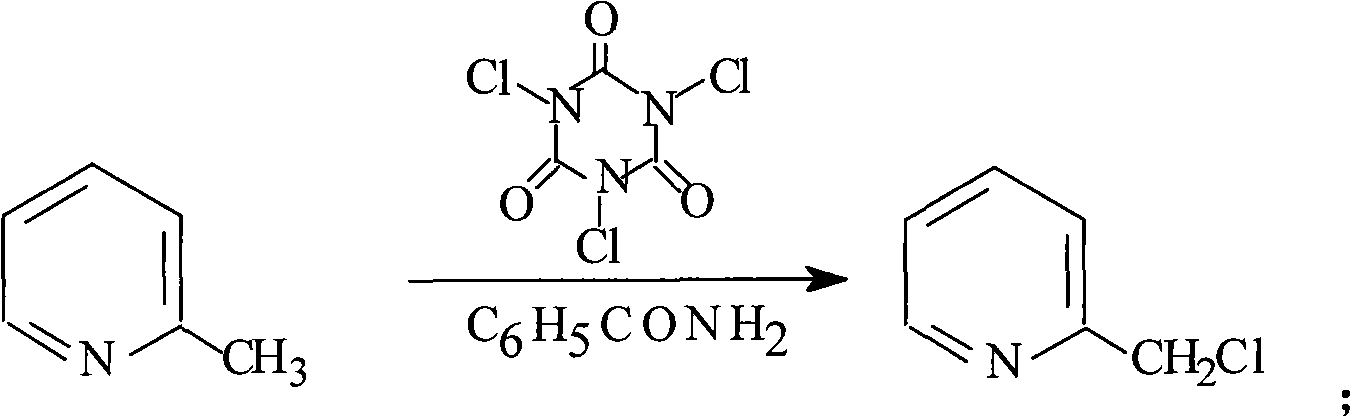
[0031] Embodiment 1: the preparation of 2-chloromethylpyridine (3)
[0032] In a 250 mL three-necked flask, 2-picoline (2) (33 g, 0.35 mol), chloroform (125 mL), and benzamide (1.5 g, 12.3 mmol) were sequentially added. Raise the temperature to reflux, add the chlorinating agent trichloroisocyanate (90g, 0.38mol) within 1h, continue to heat up to 40°C and reflux for 2h, track and analyze by thin-layer chromatography, when the reaction is completely converted into 2-chloromethylpyridine ,cool down. The reaction solution was filtered, the filter residue was washed with chloroform (2×100 mL), and the washings were combined. The organic phase was washed with saturated sodium carbonate solution, the layers were separated, the aqueous layer was extracted with chloroform (2×50 mL), the organic phases were combined, and the solvent was evaporated under reduced pressure to obtain 42.4 g of 2-chloromethylpyridine (3), with a yield of 95% . 1 HNMR (400MHz, CDCl 3 ): δ5.8 (s, 2H), 7.3...
Embodiment 2
[0033] Embodiment 2: the preparation of 2-chloromethylpyridine (3)
[0034] In a 250 mL three-necked flask, 2-picoline (2) (33 g, 0.35 mol), dichloromethane (125 mL), and benzamide (1.5 g, 12.3 mmol) were sequentially added. Raise the temperature to reflux, add the chlorinating agent trichloroisocyanate (90g, 0.38mol) within 1h, continue to heat up to 90°C and reflux for 2.5h, follow up and analyze by thin layer chromatography, until the reaction is completely converted into 2-chloromethylpyridine , cool down. The reaction solution was filtered, the filter residue was washed with chloroform (2×100 mL), and the washings were combined. The organic phase was washed with saturated sodium carbonate solution, the layers were separated, the aqueous layer was extracted with dichloromethane (2×50 mL), the organic phases were combined, and the solvent was evaporated under reduced pressure to obtain 42.3 g of 2-chloromethylpyridine (3). The yield was 94%. 1 HNMR (400MHz, CDCl 3 ): δ5...
Embodiment 3
[0035] Embodiment 3: the preparation of 2-chloromethylpyridine (3)
[0036] In a 250 mL three-necked flask, 2-picoline (2) (33 g, 0.35 mol), 1,2-dichloroethane (125 mL), and benzamide (1.5 g, 12.3 mmol) were sequentially added. Raise the temperature to reflux, add the chlorinating agent trichloroisocyanate (90g, 0.38mol) within 1h, continue to heat up to 60°C and reflux for 3h, follow up and analyze by thin layer chromatography, when the reaction is completely converted into 2-chloromethylpyridine ,cool down. The reaction solution was filtered, the filter residue was washed with chloroform (2×100 mL), and the washings were combined. The organic phase was washed with saturated sodium carbonate solution, the layers were separated, the aqueous layer was extracted with chloroform (2×50 mL), the organic phases were combined, and the solvent was evaporated under reduced pressure to obtain 40.1 g of 2-chloromethylpyridine (3), with a yield of 90% . 1 HNMR (400MHz, CDCl 3 ): δ5.8 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com