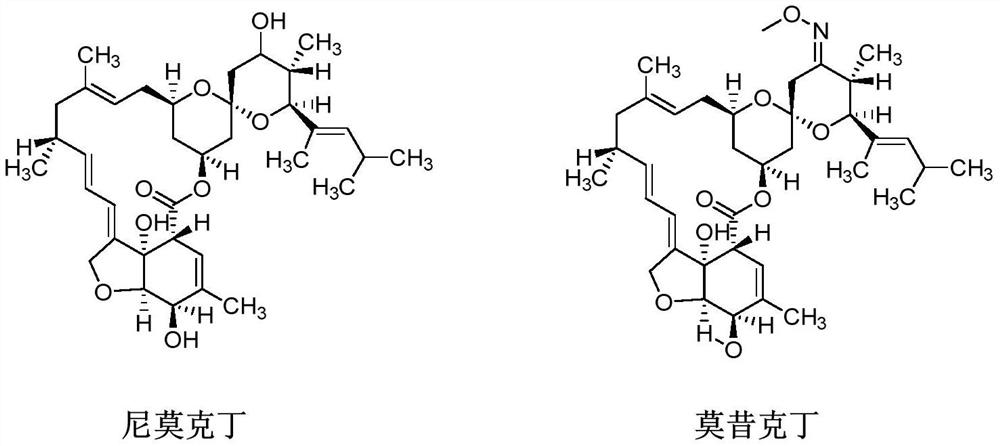
A kind of method of synthesizing moxidectin
A technology for moxidectin and intermediates, which is applied in the field of synthesizing moxidectin, which can solve the problem of low purity and yield of oxidized intermediates and final products of moxidectine, which affects the health of operators and the cost of environmental protection for enterprises Advanced problems, to achieve the effect of easy separation and purification, low cost, and cost control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
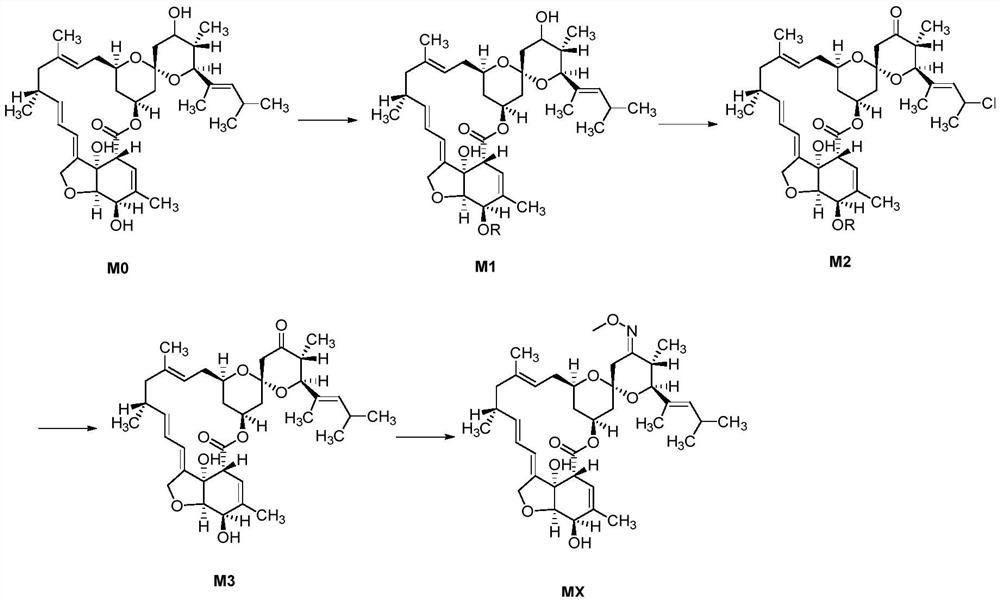
[0035] The present embodiment provides a method for synthesizing moxidectin, comprising the steps of:
[0036] 1) Add nimoctine (50g, purity 61%, 49.7mmol, 1.0eq), dichloromethane (500mL) and acid-binding agent triethylamine (30.2g, 298.5mmol, 6.0eq) in the there-necked flask, stir After nitrogen replacement, the temperature was lowered to -10~0°C under the protection of nitrogen, and the dichloromethane (100mL) solution of 4-nitrobenzoyl chloride (23.1g, 124.25mmol, 2.5eq) was added dropwise to react. The 3'hydroxyl group of moctin M0 is protected, and the internal temperature is controlled from -10 to 0°C.
[0037] After the reaction is complete, add 25 mL of methanol dropwise to the reaction liquid to quench, add 150 mL of water, let stand to separate the liquid, wash the organic layer with 1% hydrochloric acid and 15% saline successively, dry over anhydrous sodium sulfate, filter, and the filtrate is precipitated under negative pressure , adding 250 mL of methanol for cry...
Embodiment 2
[0043] The present embodiment provides a method for synthesizing moxidectin, comprising the steps of:
[0044] 1) This step is similar to step 1) of Example 1, the only difference is that the acid-binding agent triethylamine is replaced by imidazole (20.3g, 298.5mmol, 6.0eq) of the same molar mass.
[0045] As a result, 41.5 g of the upper protected intermediate M1 powder was obtained (yield 88.0%, HPLC purity 80.4%).
[0046] 2) Add 200 mL of toluene, Dess Martin oxidant (68.5 g, 161.4 mmol, 3.0 eq) and acid-binding agent potassium carbonate (18.6 g, 134.5 mmol, 2.5 eq) into a three-necked flask. The temperature was lowered to 10±5°C, and 120 mL of toluene solution of protected intermediate M1 powder (41 g, 53.8 mmol, 1.0 eq) was added dropwise, and the reaction temperature was controlled at 10±5°C.
[0047] After the feeding is completed, after the reaction at 10±5°C, the saturated sodium thiosulfate solution is added dropwise to terminate the reaction, and the liquid is se...
Embodiment 3
[0051] The present embodiment provides a method for synthesizing moxidectin, comprising the steps of:
[0052] 1) This step is similar to the step 1) of Example 1, except that dichloromethane is replaced by equal-volume 2-methyltetrahydrofuran, and the acid-binding agent triethylamine is replaced by pyridine of the same molar mass ( 23.6 g, 298.5 mmol, 6.0 eq).
[0053] As a result, 42.0 g of the upper protected intermediate M1 powder was obtained (yield 91.5%, HPLC purity 82.6%).
[0054] 2) Add 210 mL of 2-methyltetrahydrofuran, Dess Martin oxidizer (46.7 g, 110.2 mmol, 2.0 eq), and sodium hydroxide (11.0 g, 275.5 mmol, 5.0 eq) into a three-necked flask. The temperature was raised to 50±5°C, and 120 mL of 2-methyltetrahydrofuran solution of protected intermediate M1 powder (42 g, 55.1 mmol, 1.0 eq) was added dropwise, and the reaction temperature was controlled at 50±5°C.
[0055] After the feeding is completed, keep warm at 50±5°C. After the reaction is completed, add sat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com