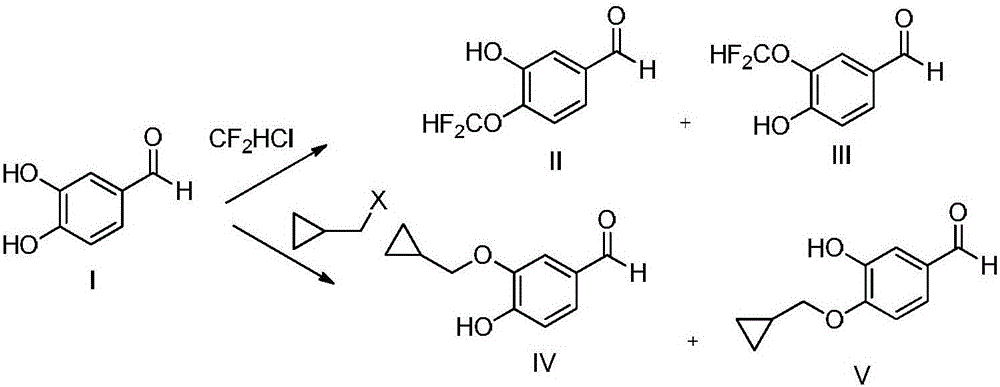
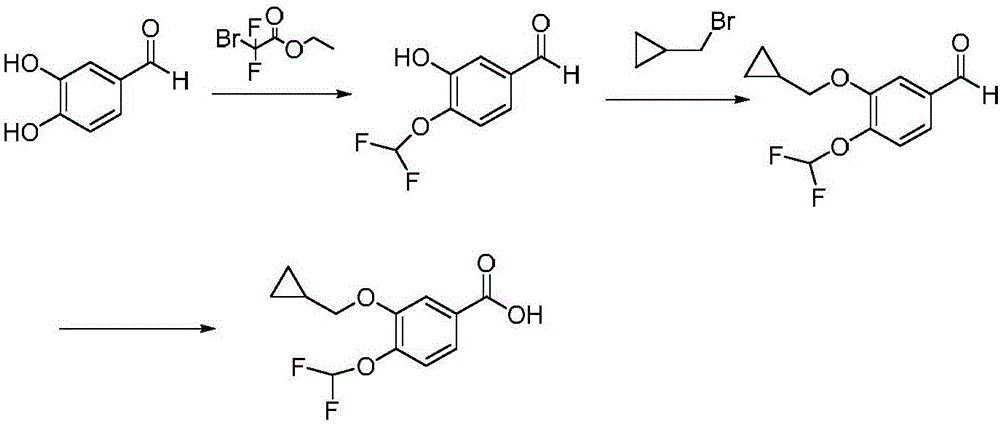
A kind of preparation method of roflumilast intermediate
A technology of roflumilast and intermediates, which is applied in the direction of oxidative preparation of carboxylic acids, organic chemistry, etc., can solve the problems of low selectivity, low reaction yield, difficult purification, etc., and achieve improved yield, less by-products, Effect of Yield Improvement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] A preparation method of roflumilast intermediate, the preparation method comprising:
[0031] 1) Add hydroxylamine (50wt% aqueous solution) (16.5g), 3,4-dihydroxybenzaldehyde (60.8g, 500mmol), methyl difluorobromoacetate (106.6g, 525mmol) into acetonitrile at 45°C for contact After the reaction, the reaction solution was added with water, extracted with dichloromethane, concentrated, and recrystallized from petroleum ether to obtain 84.9 g of 3-hydroxy-4-difluoromethoxybenzaldehyde, with a yield of 90.3% and a purity of 98.66%.
[0032] 2) 3-hydroxy-4-difluoromethoxybenzaldehyde (37.6g, 200mmol), bromomethylcyclopropane (29.7g, 220mmol) and N-methylmorpholine (28.3g, 280mmol) and zinc nitrate (22.7g, 120mol) in acetonitrile for a mixed reaction, the temperature of the mixed reaction is 55 ° C, after the reaction, the reaction solution is added with water, dichloromethane extraction, concentration, methanol recrystallization to obtain 3-cyclopropane 42.5 g of methoxy-4-...
Embodiment 2
[0035] A preparation method of roflumilast intermediate, the preparation method comprising:
[0036] 1) Add hydroxylamine (50wt% aqueous solution) (13.2g), 3,4-dihydroxybenzaldehyde (60.8g, 500mmol), methyl difluorobromoacetate (106.6g, 525mmol) into acetonitrile at 45°C for contact After the reaction, the reaction solution was added with water, extracted with dichloromethane, concentrated, and recrystallized from petroleum ether to obtain 84.4 g of 3-hydroxy-4-difluoromethoxybenzaldehyde, with a yield of 89.7% and a purity of 99.23%.
[0037]2) 3-hydroxy-4-difluoromethoxybenzaldehyde (37.6g, 200mmol), bromomethylcyclopropane (29.7g, 220mmol) and N-methylmorpholine (26.3g, 260mmol) and zinc nitrate (30.3g, 160mol) in acetonitrile for mixed reaction, the temperature of the mixed reaction is 60 ° C, after the reaction, the reaction solution is added with water, dichloromethane extraction, concentration, methanol recrystallization to obtain 3-cyclopropane 42.9 g of methoxy-4-dif...
Embodiment 3
[0040] A preparation method of roflumilast intermediate, the preparation method comprising:
[0041] 1) Add hydroxylamine (50wt% aqueous solution) (19.8g), 3,4-dihydroxybenzaldehyde (60.8g, 500mmol), methyl difluorobromoacetate (111.6g, 550mmol) into acetonitrile at 45°C for contact After the reaction, the reaction solution was added with water, extracted with dichloromethane, concentrated, and recrystallized from petroleum ether to obtain 84.2 g of 3-hydroxy-4-difluoromethoxybenzaldehyde, with a yield of 89.5% and a purity of 99.08%.
[0042] 2) 3-hydroxy-4-difluoromethoxybenzaldehyde (37.6g, 200mmol), bromomethylcyclopropane (28.4g, 210mmol) and N-methylmorpholine (30.3g, 300mmol) and zinc nitrate (26.5g, 140mol) in acetonitrile for mixed reaction, the temperature of the mixed reaction is 55 ° C, after the reaction, the reaction solution is added with water, dichloromethane extraction, concentration, methanol recrystallization to obtain 3-cyclopropane 41.5 g of methoxy-4-di...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com