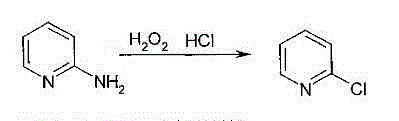
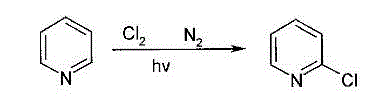
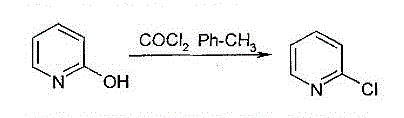Method for producing 2-chloropyridine and 2,6-chloropyridine through organic solvent method
A technology for organic solvent and dichloropyridine, which is applied in the production of 2-chloropyridine and 2 fields by organic solvent method, can solve the problems of low economic benefit, by-products, difficult source of 2-hydroxypyridine, etc., achieves high economic value and is easy to use. separation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Mix pyridine and carbon tetrachloride at a mass ratio of 1.5:1 to prepare 20Kg of pyridine solution, and vaporize the pyridine solution to obtain a vapor; mix the vapor and chlorine at a mass ratio of 1:1 Finally, the feed rate of 4Kg / h is sent to the kettle-type quartz reactor to make it react under the irradiation of ultraviolet lamps. The reaction pressure is controlled at -0.5 to -1.0KPa, the reaction temperature is controlled at 190-200 ° C, and the reaction is completed. After cooling, the reaction solution was obtained; the reaction solution was distilled to remove carbon tetrachloride, and then rectified under reduced pressure under the condition of -0.08 to -0.085MPa, and the fractions with a temperature range of 110-113°C were collected to obtain colorless 2-chloropyridine 3.1 Kg (purity 99.2%), collect fractions in the range of 142-146°C, white 2,6-dichloropyridine 16.8Kg (purity 99.0%), total yield 92.7%.
Embodiment 2
[0031] Mix pyridine and carbon tetrachloride at a mass ratio of 0.5:1 to prepare 20Kg of pyridine solution, and vaporize the pyridine solution to obtain a vapor; mix the vapor and chlorine at a mass ratio of 3:1 Finally, the feed rate of 7Kg / h is sent to the kettle-type quartz reactor to make it react under the irradiation of ultraviolet lamps. The reaction pressure is controlled at -0.5 to -1.0KPa, the reaction temperature is controlled at 180-210°C, and the reaction is completed. After cooling, the reaction solution was obtained; the reaction solution was distilled to remove carbon tetrachloride, and then rectified under reduced pressure under the condition of -0.08 to -0.085MPa, and the fractions with a temperature range of 110-113°C were collected to obtain colorless 2-chloropyridine 8.0 Kg (purity 99.6%), the fractions in the range of 142-146°C were collected to obtain 0.7Kg of white 2,6-dichloropyridine (purity 99.8%), and the total yield was 90.0%.
Embodiment 3
[0033] Mix pyridine and carbon tetrachloride at a mass ratio of 0.5:1 to prepare 20Kg of pyridine solution, and vaporize the pyridine solution to obtain a vapor; mix the vapor and chlorine at a mass ratio of 2.5:1 Finally, the feed rate of 7Kg / h is sent to the kettle-type quartz reactor to make it react under the irradiation of ultraviolet lamps. The reaction pressure is controlled at -0.5 to -1.0KPa, the reaction temperature is controlled at 180-210°C, and the reaction is completed. After cooling, the reaction liquid was obtained; the reaction liquid was distilled to remove carbon tetrachloride, and then rectified under reduced pressure under the condition of -0.08 to -0.085 MPa, and the fractions with a temperature range of 110-113 ° C were collected to obtain colorless 2-chloropyridine 7.1 Kg (purity 99.6%), the fractions in the range of 142-146°C were collected to obtain 2.7Kg of white 2,6-dichloropyridine (purity 99.8%), and the total yield was 97.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com