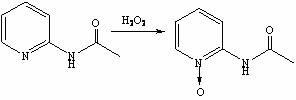Method for synthesis preparation of 2-chloro-4-aminopyridine
A technology of aminopyridine and acetamidopyridine, applied in the field of preparation of 2-chloro-4-aminopyridine, can solve the problems of unstable method, complicated reaction conditions, expensive and difficult raw materials, etc., and achieves simple operation, easy-to-obtain raw materials, The effect of high overall yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
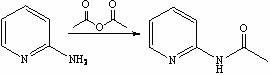
[0026] Synthesis of step 1, 2-acetylaminopyridine
[0027] Add 2-aminopyridine (23.6 g, 0.250 mol) into the reactor, add acetic anhydride (166 mL, 0.675 mol) dropwise under stirring, raise the temperature to 45-50 °C and continue the reaction for 2 h, TLC detects that the reaction of the raw materials is complete, stop the reaction , cooled to 20~25 ℃ and directly enter the next reaction;
[0028] Synthesis of step 2, 2-acetylaminopyridine nitrogen oxide
[0029] Add 30wt.% hydrogen peroxide (50 mL, 0.438 mol) dropwise to the acetylation reaction solution obtained in step 1, and react at 45-50 °C for 2.5 h. TLC detects that the reaction of the raw material 2-acetylaminopyridine is complete, and the reaction is stopped;
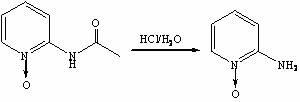
[0030] Step 3, the synthesis of 2-aminopyridine nitrogen oxide
[0031] Add 25wt.% hydrochloric acid aqueous solution (69 mL, 0.575 mol) to the oxidation reaction solution obtained in step 2, raise the temperature to 80-90°C, and react for 2.5 hours. TLC det...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com